Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
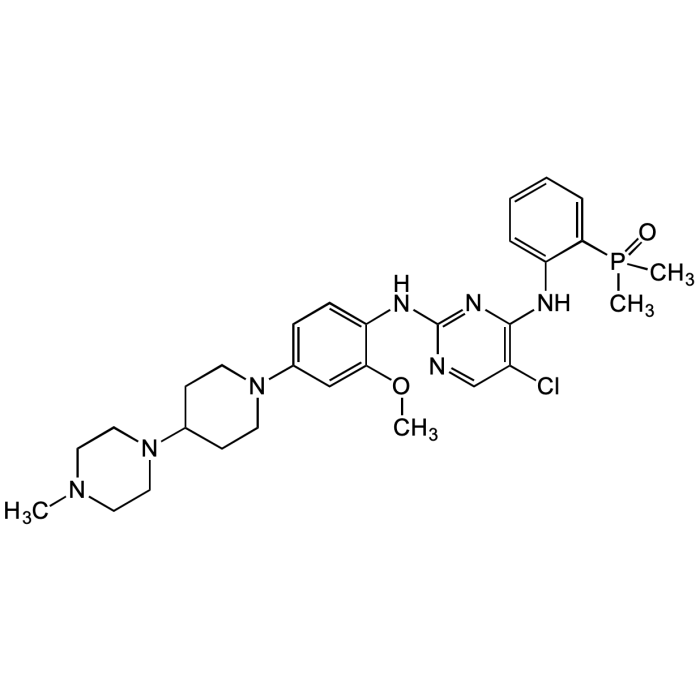
Brigatinib
| Product Details | |
|---|---|
| Synonyms | AP26113; 5-Chloro-N4-[2-(dimethylphosphinyl)phenyl]-N2-[2-methoxy-4-[4-(4-methyl-1-piperazinyl)-1-piperidinyl]phenyl]-2,4-pyrimidinediamine |
| Product Type | Chemical |
| Properties | |
| Formula | C29H39ClN7O2P |
| MW | 584.09 |
| CAS | 1197953-54-0 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to yellow solid. |
| Solubility | Soluble in DMSO. Sligthly soluble in ethanol. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AILRADAXUVEEIR-UHFFFAOYSA-N |
| Smiles | CN1CCN(C2CCN(C3=CC=C(NC4=NC(NC5=CC=CC=C5P(C)(C)=O)=C(Cl)C=N4)C(OC)=C3)CC2)CC1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Brigitanib acts as a tyrosine kinase inhibitor with activity against multiple kinases including ALK, ROS1, insulin-like growth factor 1 receptor and against EGFR deletions and point mutations. It acts by inhibiting ALK phosphorylation and the activation of downstream signaling proteins. It is a highly potent and selective inhibitor of anaplastic lymphoma kinase (ALK). Blocks ALK activity and reduces growth in neuroblastoma cells, mouse xenograft, and Drosophila model systems harboring constitutively active ALK variants. It is potentially useful for the treatment of non-small cell lung cancer (NSCLC). Can be used in combination with anti-EGFR drugs to overcome EGFR mutation resistance.
(1) R. Katayama, et al.; PNAS 108, 7535 (2011) | (2) M. Ceccon, et al.; Mol. Cancer Res. 11, 122 (2013) | (3) J.T. Siaw, et al.; Oncotarget 7, 29011 (2016) | (4) W.S. Huang, et al.; J. Med. Chem. 59, 4948 (2016) | (5) S. Zhang, et al.; Clin. Cancer Res. 22, 5527 (2016) | (6) K. Uchibori, et al.; Nat. Commun. 8, 14768 (2017) | (7) L. Mezquita & D. Planchard; Cancer Manag. Res. 10, 123 (2018) | (8) S.A. Spencer, et al.; Ann. Pharmacother. 53, 621 (2019)





