Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
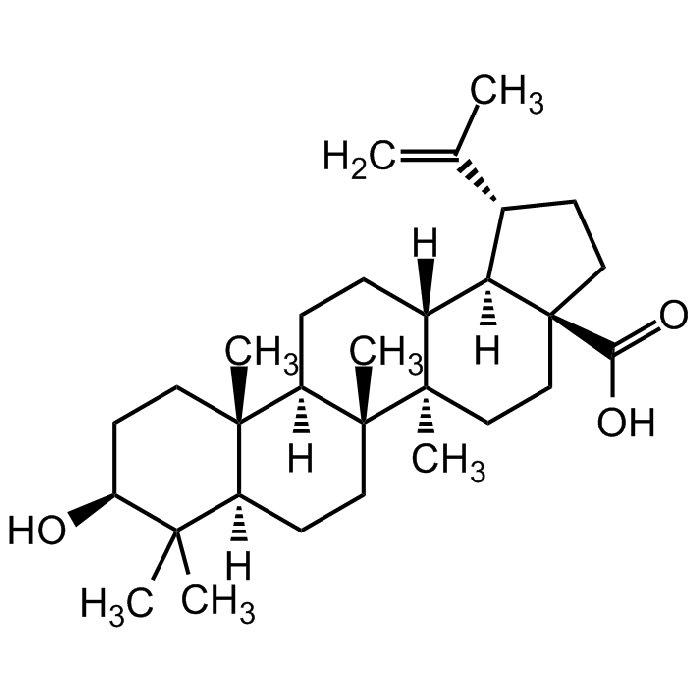
Betulinic acid
| Product Details | |
|---|---|
| Synonyms | 3β-Hydroxy-20(29)-lupaene-28-oic acid |
| Product Type | Chemical |
| Properties | |
| Formula |
C30H48O3 |
| MW | 456.71 |
| CAS | 472-15-1 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in DMSO (20mg/ml) or DMF (15mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QGJZLNKBHJESQX-FZFNOLFKSA-N |
| Smiles | O[C@H]1CC[C@@]2(C)[C@](CC[C@]3(C)[C@]2([H])CC[C@@]4([H])[C@@]3(C)CC[C@]5(C(O)=O)[C@]4([H])[C@H](C(C)=C)CC5)([H])C1(C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Betulinic acid is a natural plant triterpenoid. It has been shown to have anticancer, apoptotic, anti-inflammatory, antiobesity, antimalarial and anti-HIC properties. It has been described as a potent DNA topoisomerase II inhibitor and proteasome activator. It is an agonist of the G protein-coupled bile acid receptor TGR5, which might be linked with its ability to alter gene expression and fatty liver development. Betulinic acid also reduces NF-κ signaling and expression of microRNA-33 in LPS-treated macrophages, increasing levels of the ATP-binding cassette (ABC) cholesterol transporter ABCA1.
(1) T. Fujioka, et al.; J. Nat. Prod. 57, 243 (1994) | (2) E. Pisha, et al.; Nat. Med. 1, 1046 (1995) | (3) Y. Kashiwada, et al.; J. Med. Chem. 39, 1016 (1996) | (4) Y. Takada & B.B. Aggarwal: J. Immunol. 171, 3278 (2003) | (5) H. Kasperczyk, et al.; Oncogene 24, 6945 (2005) | (6) S. Wada & R. Tanaka; Chem. Biodivers. 2, 689 (2005) | (7) L. Huang, et al.; FEBS Lett. 581, 4955 (2007) | (8) M.S. de Sa, et al.; Parasitol. Res. 105, 275 (2009) | (9) F.B. Mullauer, et al.; Anticancer Drugs 21, 215 (2010) | (10) C. Genet, et al.; J. Med. Chem. 53, 178 (2010) | (11) Y. Wan, et al.; Intern. Immunopharmacol. 17, 184 (2013) | (12) G.J. Zhao, et al.; PLoS One 8, e474782 (2013) | (13) K.D. Kim, et al.; Nutr. Metab. Cardiovasc. Dis. 29, 409 (2019) | (14) Y. Zheng, et al.; Oxid. Med. Cell Longev. 2019, 8781690 (2019) | (15) W. Liu, et al.; Am. J. Transl. Res. 11, 6952 (2019)





