Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
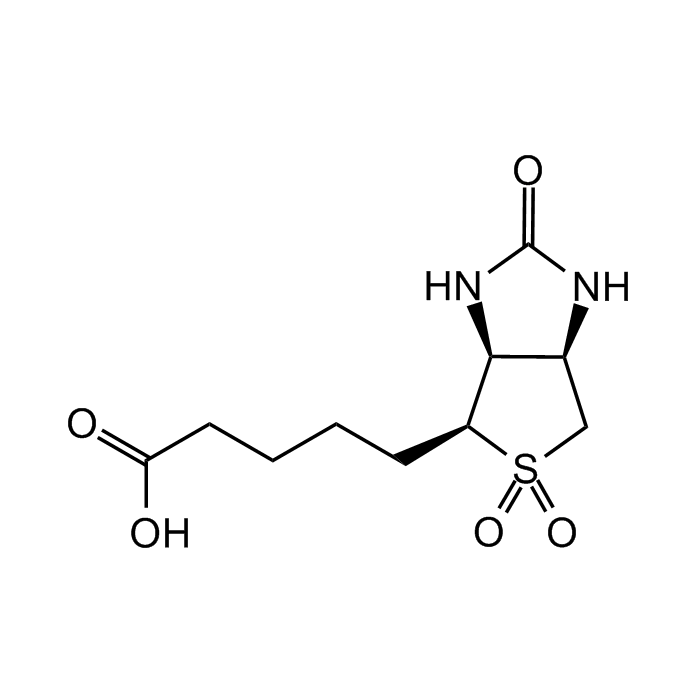
Biotin Sulfone
| Product Details | |
|---|---|
| Synonyms | Biotin 5,5-Dioxide; (3aS,4S,6aR)-hexahydro-2-oxo-1H-thieno[3,4-d]imidazole-4-pentanoic acid, 5,5-dioxide |
| Product Type | Chemical |
| Properties | |
| Formula |
C10H16N2O5S |
| MW | 276.31 |
| CAS | 40720-05-6 |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | Colorless needles. |
| Solubility | Slightly soluble in aqueous base or DMSO (<1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QPFQYMONYBAUCY-ZKWXMUAHSA-N |
| Smiles | O=C1N[C@@H]2[C@@H]([C@H](CCCCC(O)=O)S(C2)(=O)=O)N1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Biotin sulfone is an oxidized form of biotin and is as efficient as biotin in streptavidin binding and it binds to avidin with a binding ratio of 0.332 compared with biotin. Biotinylated synthetic oligosaccharides are an attractive tool, as streptavidin coated plates and beads are commercially available and widely use in serological assays. As biotin is chemically reactive, and not compatible with carbohydrate protecting group manipulation, the use of biotin sulfone tagged oligomannosides (BSTO) for antigen immobilization in candidiasis or allergy diagnosis has been used. The use of biotin sulfone also offers a convenient solution for both oligosaccharide synthesis and immobilization on microspheres and surface plasmon resonance sensors.
(1) J. Zempleni & D.M. Mock; J. Nutr. 129, 494S (1999) | (2) M. Collot, et al.; J. Med. Chem. 51, 6201 (2008) | (3) G. Despras, et al.; Bioorg. Med. Chem. 20, 1817 (2012)





