Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
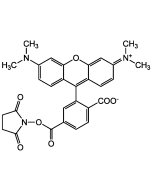
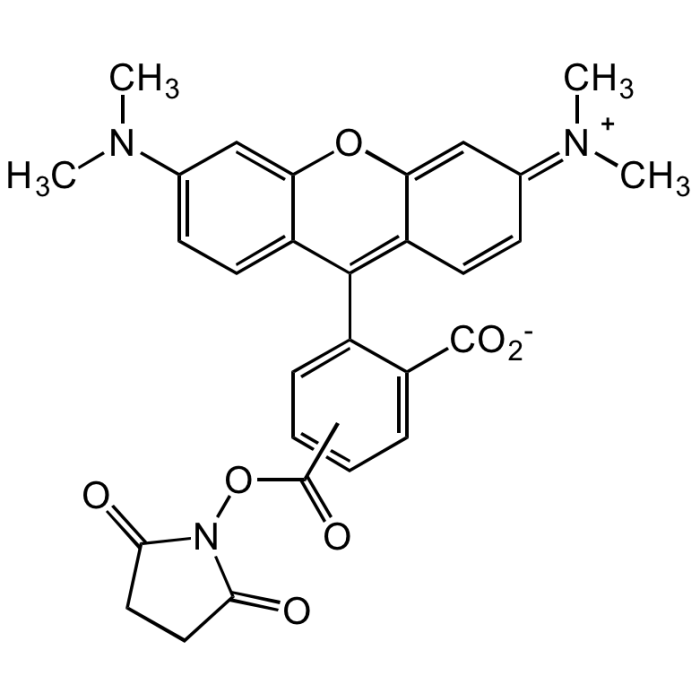
5(6)-TAMRA N-succinimidyl ester
| Product Details | |
|---|---|
| Synonyms | 5(6)-TAMRA SE; 5(6)-Carboxytetramethylrhodamine NHS; 5(6)-Carboxytetramethylrhodamine N-succinimidyl ester |
| Product Type | Chemical |
| Properties | |
| Formula |
C29H25N3O7 |
| MW | 527.52 |
| CAS | 150408-83-6 | 246256-50-8 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥90% (HPLC) |
| Appearance | Dark brown powder. |
| Solubility | Soluble in DMSO, DMF, acetonitrile or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | MCDQUPOEOXETSJ-UHFFFAOYSA-N |
| Smiles | CN(C)C1=CC=C(C(C2=CC=CC=C2C([O-])=O)=C(C=C/3)C(O4)=CC3=[N+](C)\C)C4=C1.O=C(C)ON5C(CCC5=O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Amine-reactive form of 5(6)-carboxytetramethylrhodamine. Conjugates yield bright, pH-insensitive orange-red fluorescence (Ex/Em: ~543/576nm i MeOH) with good photostability. Fluorophores most often used for preparing peptide, protein, nucleotide and nucleic acid conjugates or other primary amine-containing molecules. Especially also used for fluorescent antibodies and avidin derivatives used in immunochemistry.
(1) E. Koller Appl. Fluoresc. Technol. 3, 20, (1991) | (2) L.D. Mayfield, D.R. Corey Bioorg. Med. Chem. Lett. 9, 14, (1999) | (3) M. Hahn, et al.; Electrophoresis 22, 2691 (2001) | (4) T.M. Hsu, et al.; Clin. Chem. 47, 1373 (2001) |(5) C. Olivier, et al.; Bioconjug. Chem 14, 430 (2003) | (6) H.Y. Li, et al.; J. Cell Biol. 160, 635 (2003) | (7) W. Erker, et al.; BBRC 324, 893 (2004)