Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
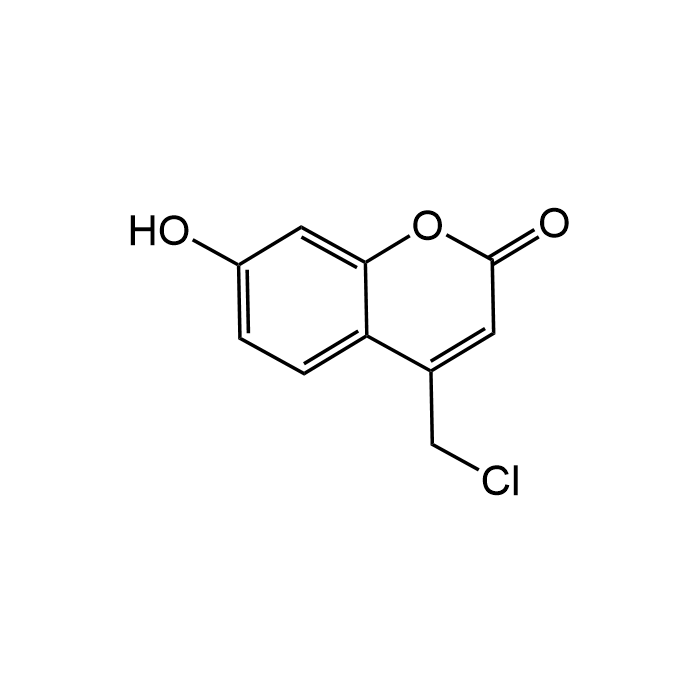
4-Chloromethyl-7-hydroxycoumarin
| Product Details | |
|---|---|
| Synonyms | Blue CMHC; 4-(Chloromethyl)-7-hydroxy-2H-chromen-2-one; Celltracker Blue CMHC |
| Product Type | Chemical |
| Properties | |
| Formula | C10H7ClO3 |
| MW | 210.61 |
| CAS | 25392-41-0 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥96% |
| Appearance | Yellow powder. |
| Solubility | Soluble in DMSO (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | TXSLBPGPBNGHRW-UHFFFAOYSA-N |
| Smiles | OC1=CC=C(C(CCl)=CC(O2)=O)C2=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
4-Chloromethyl-7-hydroxycoumarin, also known as "Celltracker Blue CMHC", is a fluorescent thiol-reactive cell permeant probe that easily pass through cell membranes and is used for tracking live cells or cellular processes. Cell movement and location studies require specialized probes that are nontoxic to living cells and well retained, allowing for multigenerational tracking. The Blue CMHC fluorescent probe can be used for co-staining with antibodies or other cell analysis probes. The dye is an excellent tool for monitoring cell movement, location, proliferation, migration, chemotaxis, and invasion. Blue CMHC is a fluorescent probe that is retained in living cells through several generations. The probe Blue CMHC is inherited by daughter cells after cell fusion and is not transferred to adjacent cells in a population. Blue CMHC can be loaded into cells by adding the reagent to the culture medium and then washing the cells briefly with fresh medium before analysis. It passes freely through cell membranes, but once inside the cell, it is transformed into a cell-impermeant reaction product. Blue CMHC contains a chloromethyl group that reacts with thiols, probably in a glutathione S-transferase-mediated reaction. Blue CMHC is transformed into a cell-impermeant fluorescent dye-thioether adduct that can be fixed with aldehyde fixatives, permitting long-term sample storage. Excess unconjugated reagent passively diffuses to the extracellular medium. The impermeable reaction product of the Blue CMHC has excellent retention, strong fluorescence and relatively uniform cytoplasmic staining, making them potentially useful for correcting motion artifacts in imaging. Cells stained with Blue CMHC are brightly fluorescent for at least 72 hours after incubation in fresh medium at 37°C and through at least four cell divisions (retention time within the cell can vary dependent upon the cell type, incubation conditions and other factors and should be empirically determined prior to any tracing experiments). No other permeant dyes of this type, including the widely used calcein AM and BCECF-AM, are retained in viable cells for more than a few hours at such physiological temperatures. Spectral data: λex 372nm; λem 470nm (Aqueous Buffer pH 10). Blue CMHC is also used as an intermediate for the development of different specific fluorescent dyes. Staining protocol: Prepare stock solution at 10mM in DMSO. Dilute the stock solution to a final working concentration of 0.5-25 µM in serum-free medium. Avoid amine- and thiolcontaining buffers. Incubate for 15-45 minutes at 37°C, replace with fresh media and incubate for 30 minutes at 37°C. Wash with PBS before imaging or fixing. Ex/Em = 372/470 nm.
(1) G. Zagotto, et al.; Photochem. Photobiol. 58, 486 (1993) | (2) C.D. Nelson, et al.; BioTechniques 29, 874 (2000) | (3) K.M.K. Swamy, et al.; Bull. Korean Chem. Soc. 31, 3611 (2010) | (4) L. Pisani, et al.; Eur. J. Med. Chem. 70, 723 (2013) | (5) X. Xu, et al.; Anal. Meth. 8, 245 (2016) | (6) H. Sherman, et al.; Front. Immunol. 9, 857 (2018) | (7) A. Kurt, et al.; JOTCSA 8, 155 (2021)





