Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
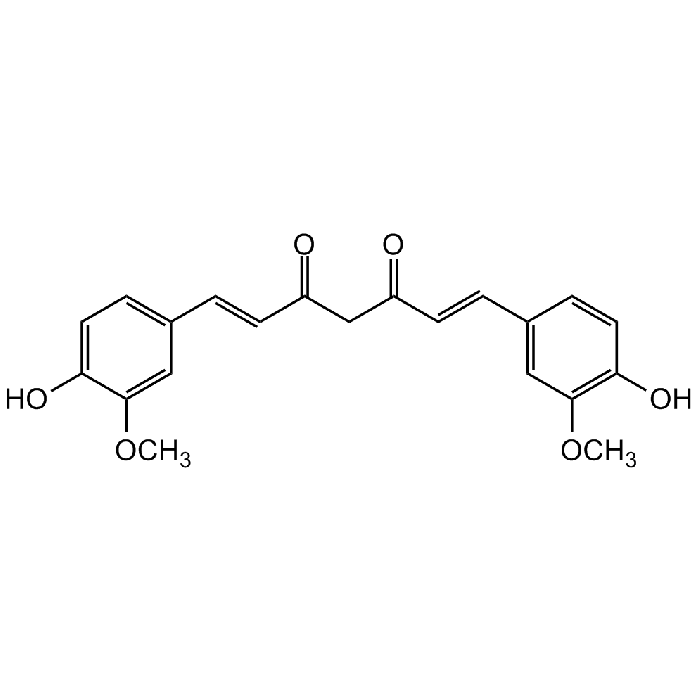
Curcumin
| Product Details | |
|---|---|
| Synonyms | (E,E)-1,7-bis(4-Hydroxy-3-methoxyphenyl)-1,6-heptadiene-3,5-dione; Diferuloylmethane; Diferulylmethane; Diferuloylmethane; Natural Yellow 3 |
| Product Type | Chemical |
| Properties | |
| Formula |
C21H20O6 |
| MW | 368.38 |
| CAS | 458-37-7 |
| RTECS | MI5230000 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥95% |
| Appearance | Yellow to orange solid. |
| Solubility | Soluble in DMSO (25mg/ml) or ethanol (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VFLDPWHFBUODDF-FCXRPNKRSA-N |
| Smiles | OC1=CC=C(/C=C/C(CC(/C=C/C2=CC(OC)=C(O)C=C2)=O)=O)C=C1OC |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Curcumin is the major yellow pigment in turmeric and curry and has antioxidant, anti-inflammatory, neuroprotective, cardioprotective, antidiabetic, antiviral, antibacterial, antifungal, chemopreventive and antitumor activities. It interferes with multiple cell signaling pathways, including cell cycle (cyclin D1 and cyclin E), apoptosis (activation of caspases and down-regulation of anti-apoptotic gene products), proliferation (HER-2, EGFR and AP-1), survival (PI3K/AKT pathway), invasion (MMP-9 and adhesion molecules), angiogenesis (VEGF), metastasis (CXCR-4), tumorigenesis and development (Shh, Gli), metabolism (PPARγ, Nrf2), epigenetics (HDACs, HATs, DNA methyltransferase I and microRNAs) and inflammation (NLRP3, TLR4, NF-κB, TNF, IL-6, IL-1, COX-2 and 5-LOX).
(1) R.K. Maheshwari, et al.; Life Sci. 78, 2081 (2006) (Review) | (2) V.P. Menon & A.R. Sudheer; Adv. Exp. Med. Biol. 595, 105 (2007) (Review) | (3) G. Kuttan, et al.; Adv. Exp. Med. Biol. 595, 173 (2007) (Review) | (4) B.B. Aggarwal & K.B. Harikumar; Int. J. Biochem. Cell Biol. 41, 40 (2009) (Review) | (5) S. Fu & R. Kurzrock; Cancer 116, 4670 (2010) (Review) | (6) L. Alappat & A.B. Awad; Nutr. Rev. 68, 729 (2010) (Review) | (7) R.B. Mythri & M.M. Bharath; Curr. Pharm. Des. 18, 91 (2012) (Review) | (8) A.A. Momtazi, et al.; Rev. Physiol. Biochem. Pharmacol. 171, 1 (2016) (Review) | (9) S.S. Soflaei, et al.; Curr. Pharm. Des. 24, 123 (2018) (Review) | (10) M. Boozari, et al.; J. Cell Physiol. 234, 12471 (2019) (Review) | (11) K.R. Kahkhaie, et al.; Inflammopharmacol. 27, 885 (2019) (Review) | (12) D.J.Den Hartogh, et al.; Nutrients 12, 118 (2020) (Review) | (13) D.J.Den Hartogh, et al.; Nutrients 12, 58 (2020) (Review) | (14) M.A. Panaro, et al.; Int. J. Mol. Sci. 21, 2299 (2020) (Review) | (15) M.R. Jennings & R.J. Parks; Viruses 12, 1242 (2020) (Review) | (16) A.Saeedi-Boroujeni, et al.; Basic. Clin. Pharmacol. Toxicol. 128, 37 (2021) (Review)





