Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
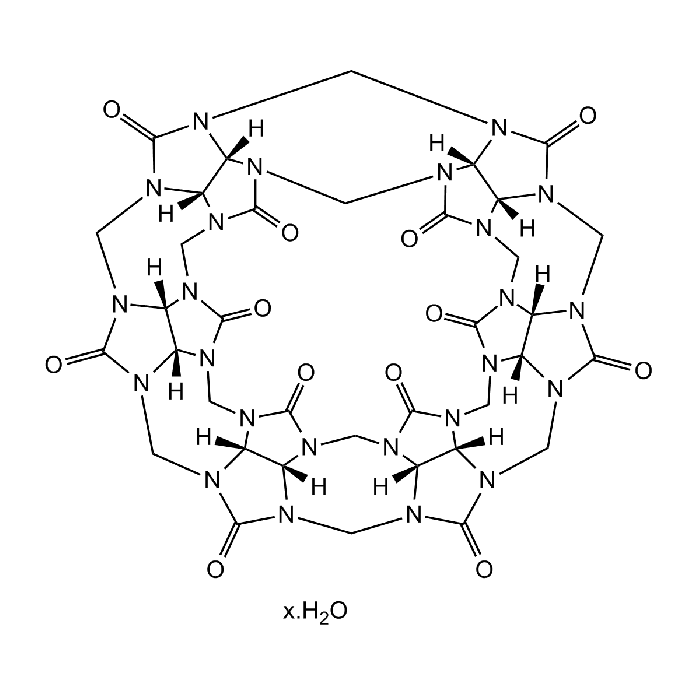
Cucurbit[6]uril hydrate
| Product Details | |
|---|---|
| Synonyms | Cucurbituril |
| Product Type | Chemical |
| Properties | |
| Formula |
C36H36N24O12 |
| MW | 996.84 |
| CAS | 80262-44-8 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (N) |
| Appearance | White to off-white powder. |
| Solubility | Very slightly soluble in water or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | MSBXTPRURXJCPF-DQWIULQBSA-N |
| Smiles | O=C(N(C1)[C@@]2([H])N3CN4[C@@]5([H])N1C(N(CN6[C@]7([H])N8C(N(C9)[C@]7([H])N(C%10)C6=O)=O)[C@@]5([H])N(C8)C4=O)=O)N(CN%11[C@@]%12([H])N%13C(N(C%14)[C@@]%12([H])N(CN%15[C@@]%16([H])N%14C(N(C%17)[C@@]%16([H])N(CN%18[C@@]%19([H])N%17C(N9[C@@]%19([H])N%10C%18=O)=O)C%15=O)=O)C%11=O)=O)[C@]2([H])N(C%13)C3=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Cucurbit[6]uril hydrate belongs to the Cucurbit[n]uril (CB[n], n = 5-10) family of macrocyclic compounds comprising n glycoluril units, self-assembled from an acid-catalyzed condensation reaction of glycoluril and formaldehyde. The pumpkin-shaped CB molecules have a hydrophobic cavity and two identical carbonyllaced portals. While the hydrophobic interior provides a potential inclusion site for nonpolar molecules, the polar ureido carbonyl groups at the portals allow CB[n] to bind ions and molecules through charge-dipole and hydrogen bonding interactions. The unique structure and recognition properties make CB[n] attractive not only as a synthetic receptor but also as a building block for the construction of supramolecular architectures. In the wide area of supramolecular chemistry, cucurbit[n]urils (CBn) present themselves as molecular containers, able to form stable complexes with various guests, including drug molecules, amino acids and peptides, saccharides, dyes, hydrocarbons, perfluorinated hydrocarbons, and even high molecular weight guests such as proteins (e.g., human insulin). Furthermore, a direct functionalization method of CB[n] allowed the synthesis of a wide variety of tailor-made CB derivatives to study many applications. Ion channels, vesicles, polymers, nanomaterials, ion selective electrodes incorporating CB[n], and CB-immobilized solid surfaces and silica gel have been reported. Numerous other applications are being explored.
(1) W.L. Mock & N.-Y. Shih; J. Org. Chem. 48, 3619 (1983) | (2) J. Zhao, et al.; Angew. Chem. Int. Ed. 40, 4233 (2001) | (3) K. Kim; Chem. Soc. Rev. 31, 96 (2002) | (4) Y.-B. Lim; et al.; Bioconjugate Chem. 13, 1181 (2002) | (5) J.W. Lee, et al.; Acc. Chem. Res. 36, 621 (2003) | (6) S.Y. Jon, et al.; JACS 125, 10186 (2003) | (7) J. Lagona, et al.; Angew. Chem. Int. Ed. 44, 4844 (2005) | (8) K. Kim, et al.; Chem. Soc. Rev. 36, 267 (2007) | (9) D. Kim, et al.; Angew. Chem. Int. Ed. 46, 3471 (2007) | (10) E. Kim, et al.; Angew. Chem. Int. Ed. 49, 4405 (2010) | (11) K.I. Assaf & W.M. Nau; Chem. Soc. Rev. 44, 394 (2015) | (12) D. Das, et al.; Front. Chem. 7, 619 (2019) | (13) R. Gao, et al.; Molecules 28, 3566 (2023) | (14) S. Shukla, et al.; ASC Appl. Bio. Mater. 6, 2089 (2023)





