Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
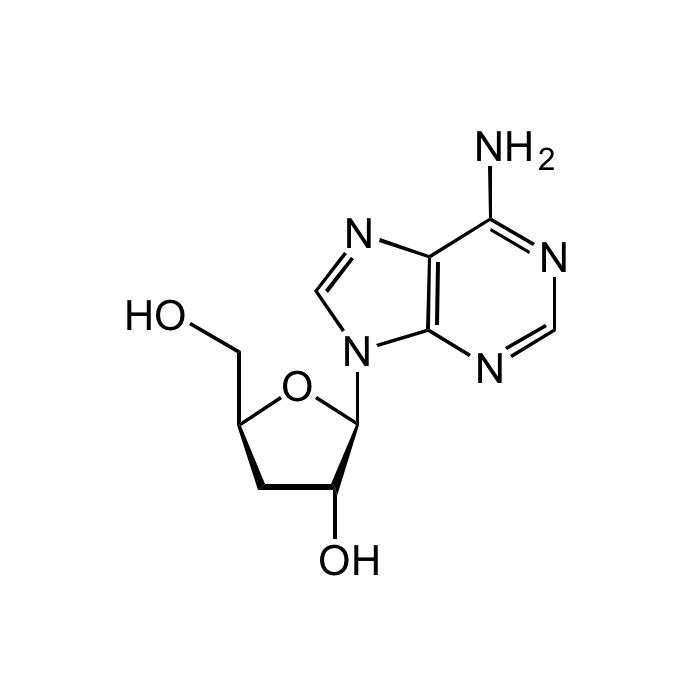
Cordycepin
| Product Details | |
|---|---|
| Synonyms | 3'-Deoxyadenosine; NSC 63984; NSC 401022; 9-Cordyceposidoadenine; BRN 0035194 |
| Product Type | Chemical |
| Properties | |
| Formula |
C10H13N5O3 |
| MW | 251.24 |
| CAS | 73-03-0 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water, ethanol or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | OFEZSBMBBKLLBJ-BAJZRUMYSA-N |
| Smiles | OC[C@@H]1C[C@@H](O)[C@H](N2C(N=CN=C3N)=C3N=C2)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Cordycepin is a nucleoside (adenosine analog) antibiotic that acts as a polyadenylation inhibitor when converted to 3’-deoxyadenosine triphosphate (3'-dATP), which inhibits ATP-dependent DNA and RNA synthesis. Cordycepin has a broad spectrum of biological activities, including anticancer, anti-proliferative, pro-apoptotic, anti-inflammatory and antifungal effects. It was shown to modulate autophagy and NLRP3 inflammasome. Cordycepin has been shown to reduce poly(A) tail length in mRNA, to activate AMPK kinase, leading to a reduction in mTOR signalling, downregulate ERK/Akt/PI3K/GSK-3β/β-catenin signaling pathways. Also useful for the study of messenger RNA transcription.
(1) G.G. Lovinger, et al.; Virology 55, 524 (1973) | (2) R.I. Gumport, et al.; Biochemistry 15, 2804 (1976) | (3) A.M. Sugar & R.P. McCaffrey; Antimicrob. Agents Chemother. 42, 1424 (1998) | (4) E.N. Kodama, et al.; Biochem. Pharmacol. 59, 273 (2000) | (5) L.S. Chen, et al.; Br. J. Haematol. 140, 682 (2008) | (6) Y.Y. Wong, et al.; J. Biol. Chem. 285, 2610 (2010) | (7) H.J. Lee, et al.; Invest. New Drugs 30, 1917 (2012) | (8) B.S. Ko, et al.; PLoS One 8, e76320 (2013) | (9) H.S. Tuli, et al.; Life Sci. 93, 863 (2013) (Review) | (10) C. Wu, et al.; J. Cell Mol. Med. 18, 293 (2014) | (11) K. Nakamura, et al.; J. Pharmacol. Sci. 127, 53 (2015) (Review) | (12) Y. Li, et al.; Oncol. Lett. 9, 2541 (2015) | (13) J. Yang, et al.; Biomed. Pharmacother. 95, 1777 (2017) | (14) Y. Wang, et al.; Exp. Ther. Med. 14, 3067 (2017)