Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
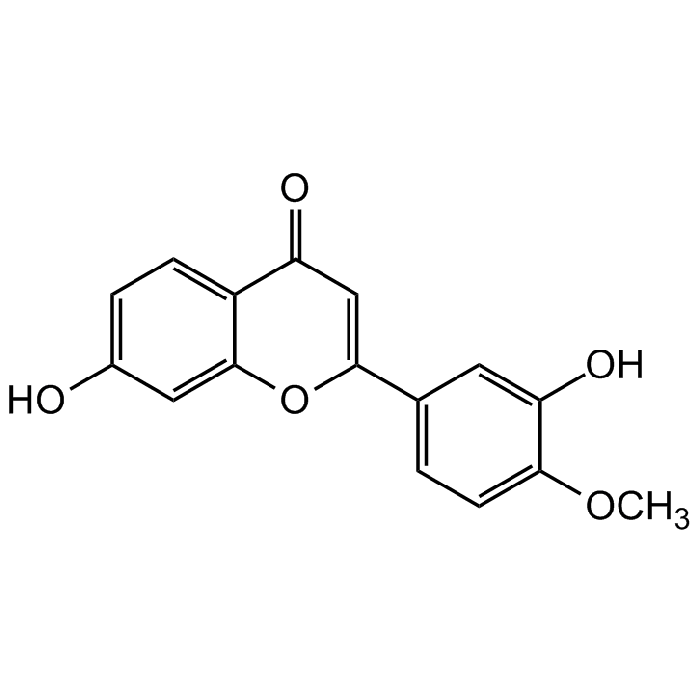
Calycosin
| Product Details | |
|---|---|
| Synonyms | 3',7-Dihydroxy-4'-methoxy-isoflavone; 3'-Hydroxyformononetin |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H12O5 |
| MW | 284.26 |
| CAS | 20575-57-9 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to light yellow powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF (20mg/ml) or methanol (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QAGGICSUEVNSGH-UHFFFAOYSA-N |
| Smiles | O=C1C=C(C2=CC=C(OC)C(O)=C2)OC3=CC(O)=CC=C31 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Calycosin is an isoflavone and phytoestrogen with diverse biological properties, including anti-cancer, anti-inflammatory, antioxidant, neuroprotective, antiplasmodial and antiprotozoal activities. It is an estrogen receptor (ER) partial agonist that inhibits 17β-estradiol binding to ERα and ER, promotion angiogenesis. It has been shown to have calcium blocking activities. Suppressed breast cancer cell growth via ERα-dependent regulation of IGF-1R, p38 MAPK and PI3K/Akt pathways. Shown to induce apoptosis in several cancer types and having antimetastatic and anti-tumor activities.
(1) C. Kraft, et al.; J. Ethnopharmacol. 73, 131 (2000) | (2) D.-H. Yu, et al.; Biomed. Environ. Sci. 18, 297 (2005) | (3) X.L. Wu, et al.; Acta Pharmacol. Sin. 27, 1007 (2006) | (4) M.M. Salem & K.A. Werbovetz; J. Nat. Prod. 69, 43 (2006) | (5) J.Y. Tang, et al.; PLoS One 5, e11822 (2010) | (6) C. Guo, et al.; J. Ethnopharmacol. 144, 768 (2012) | (7) J. Chen, et al.; PLoS One 9, e91245 (2014) | (8) R. Qiu, et al.; Exp. Mol. Pathol. 97, 17 (2014) | (9) X. Zhao, et al.; Gene 591, 123 (2016) | (10) L. Song, et al.; Neural. Regen. Res. 12, 1870 (2017) | (11) L. Dong, et al.; Gene 675, 94 (2018) | (12) R. Qiu, et al.; Biofactors 45, 975 (2019) | (13) C. Huang, et al.; Med. Sci. Monit. 25, 5589 (2019)





