Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
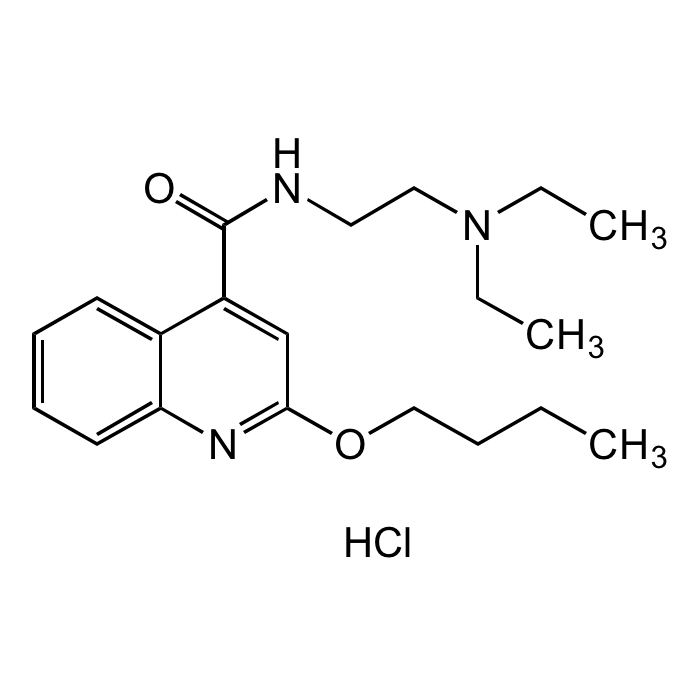
Dibucaine hydrochloride
| Product Details | |
|---|---|
| Synonyms | Cinchocaine hydrochloride; 2-Butoxy-N-(2-diethylaminoethyl)-4-quinolinecarboxamide hydrochloride |
| Product Type | Chemical |
| Properties | |
| Formula | C20H29N3O2 . HCl |
| MW | 343.4 . 36.5 |
| CAS | 61-12-1 |
| RTECS | GD3325000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water (50mg/ml), DMSO (50mg/ml) or ethanol (50mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | IVHBBMHQKZBJEU-UHFFFAOYSA-N |
| Smiles | O=C(NCCN(CC)CC)C1=CC(OCCCC)=NC2=CC=CC=C21.Cl |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Dibucaine is a long-acting local anesthetic. Potent Na+ channel blocker. It blocks both the initiation and conduction of nerve impulses by decreasing the neuronal membrane's permeability to sodium ions. This reversibly stabilizes the membrane and inhibits depolarization, resulting in the failure of a propagated action potential and subsequent conduction blockade. Induces apoptosis through calpain, cytochrome oxidase etc, and shows antiviral and antiparasitic activity.
(1) G.R. Padmanabhan, et al.; Anal. Profiles Drug Subs. 12, 105 (1983) | (2) A Seelig; Biochim. Biophys. Acta 899, 196 (1987) | (3) E. Garcia-Martin & C. Gutierrez-Merino; J. Neurochem. 54, 1238 (1990) | (4) B.K. Stringer & H.J. Harmon; Biochem. Pharmacol. 40, 1077 (1990) | (5) A. Oda, et al.; Am. J. Physiol. 269, C118 (1995) | (6) Y. Kuroda, et al.; Biophys. J. 71, 1191 (1996) | (7) M. Kim, et al.; Exp. Cell Res. 231, 235 (1997) | (8) M. Kansha, et al.; Anesthesiology 91, 1798 (1999) | (9) K. Arita, et al.; Biochem. Pharmacol. 60, 905 (2000) | (10) T. Souto-Padron, et al.; Parasitol. Res. 99, 317 (2006) | (11) W. Zhang, et al.; Int. J. Mol. Sci. 12, 2125 (2011) | (12) H.A. Douglas, et al.; J. Neurophysiol. 105, 1482 (2011) | (13) R. Ulferts, et al.; Antimicrob. Agents Chemother. 60, 2627 (2016)





