Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
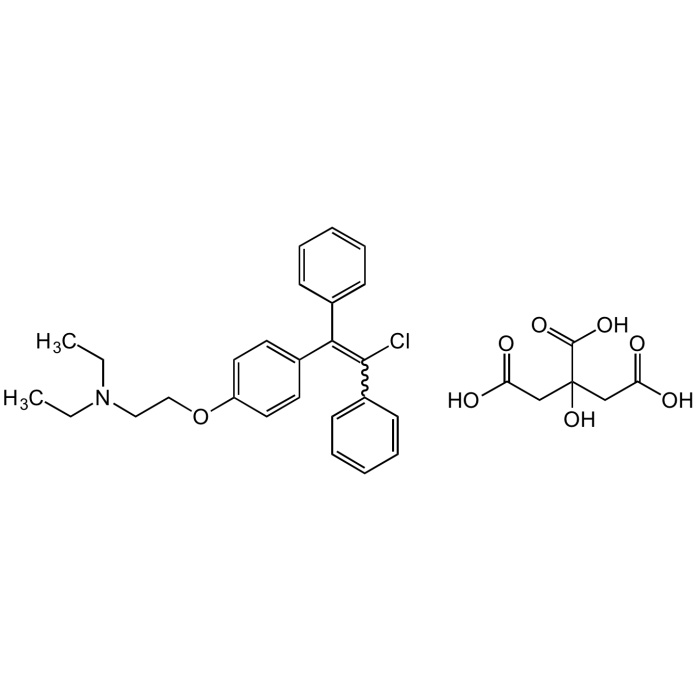
Clomiphene citrate
| Product Details | |
|---|---|
| Synonyms | Clomifene; Omifin; NSC 35770; 2-(4-[2-Chloro-1,2-diphenylethenyl]phenoxy)-N,N-diethylethanamine; Clomiphene citrate salt |
| Product Type | Chemical |
| Properties | |
| Formula |
C26H28ClNO . C6H8O7 |
| MW | 598.08 |
| CAS | 50-41-9 |
| RTECS | YE0875000 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Solid. |
| Solubility | Soluble in DMOS or DMF. Slightly soluble in water, methanol or chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PYTMYKVIJXPNBD-BTKVJIOYSA-N |
| Smiles | Cl/C(C1=CC=CC=C1)=C(C2=CC=CC=C2)/C3=CC=C(OCCN(CC)CC)C=C3.OC(CC(C(O)=O)(O)CC(O)=O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Clomiphene is a nonsteroidal triphenylethylene derivative. It acts as a selective estrogen receptor modulator (SERM), that impairs the activation of estrogen receptors (ERs) by 17β-estradiol, similar to tamoxifen and raloxifene. It potently binds both ERα and ERβ (Ki = 0.9 and 1.2nM, respectively). Clomiphene enhances the release of gonadotropin-releasing hormone, stimulating the release of follicle-stimulating hormone and luteinizing hormone, culminating in ovulation. Triphenylethylene antiestrogens may also be used as antileukemic drugs which kill cells by apoptosis mediated by oxidative stress and activation of PKC.
(1) J.H. Clark & B.M. Markaverich; Pharmacol. Ther. 15, 467 (1981) | (2) E.Y. Adashi; Fertil. Steril. 42, 331 (1984) | (3) E. Kousta, et al.; Hum. Reprod. Update 3, 359 (1997) | (4) G.G.J.M. Kuiper, et al.; Endocrinology 138, 863 (1997) | (5) T. Hayon, et al.; Anticancer Res. 19, 2089 (1999) | (6) S.G. Haskell; South Med. J. 96, 469 (2003) | (7) R. Homburg; Hum. Reprod. 20, 2043 (2005) | (8) M. Amita, et al.; Endocrinology 151, 394 (2010)





