Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
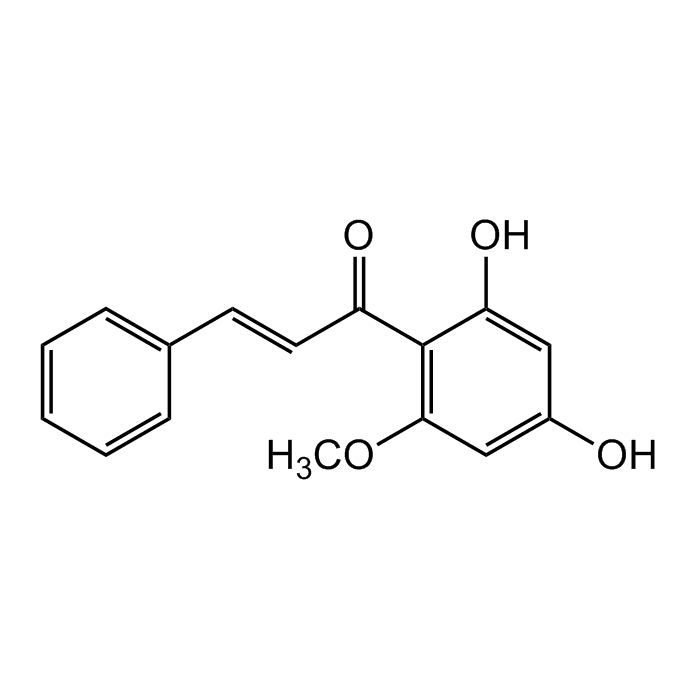
Cardamonin
| Product Details | |
|---|---|
| Synonyms | (2E)-1-(2,4-Dihydroxy-6-methoxyphenyl)-3-phenyl-2-propen-1-one; Alpinetin chalcone; (E)-2',4'-Dihydroxy-6'-methoxy-chalcone |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H14O4 |
| MW | 270.28 |
| CAS | 19309-14-9 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF (20mg/ml) or ethanol (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NYSZJNUIVUBQMM-BQYQJAHWSA-N |
| Smiles | O=C(C1=C(OC)C=C(O)C=C1O)/C=C/C2=CC=CC=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Cardamonin is a calchone from Aplinia species (zingiberaceous plant species) with anti-cancer and anti-inflammatory, anti-nociceptive activity. Cardamonin suppresses NF-κ nuclear translocation and Ik-Ba phosphorylation. It has been shown to suppress nitric oxide and prostaglandin E2 synthesis, to suppress cyclooxygenase-2 expression, and to inhibit NF-κ signaling. It induces apoptosis in cancer cells, suppresses melanogenesis by inhibition of Wnt/beta-catenin signaling and ameliorates insulin resistance. It has been shown to induce autophagy and an antiproliferative effect through JNK activation. It is an hTRPA1 antagonist and shows also anti-parasitic activity. Also shown inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway.
(1) J.H. Lee, et al.; J. Pharmacol. Exp. Ther. 316, 271 (2006) | (2) D.A. Israf, et al.; Mol. Immunol. 44, 673 (2007) | (3) M. Cho, et al.; BBRC 390, 500 (2009) | (4) V.R. Yadav, et al.; Intern. Immunopharmacol. 11, 295 (2011) | (5) Y. Qin, et al.; Leuk. Res. 36, 514 (2012) | (6) P. Niu, et al.; Planta Med. 79, 452 (2013) | (7) M.K. Park, et al.; Pharmacol. Biochem. Behav. 118, 10 (2014) | (8) N. Wu, et al.; Tumour Biol. 36, 9667 (2015) | (9) J.Y. Kim, et al.; Bioorg. Med. Chem. Lett. 25, 2559 (2015) | (10) C.C. de Castro, et al.; Phytomedicine 22, 921 (2015) | (11) S. Wang, et al.; Molecules 21, E1145 (2016) | (12) K. Wang, et al.; Biochem. Pharmacol. 155, 494 (2018)





