Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
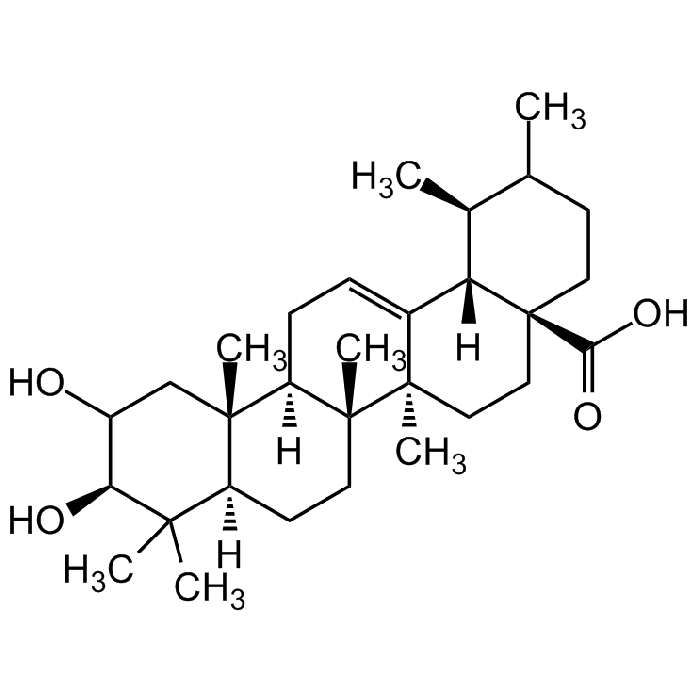
Corosolic acid
| Product Details | |
|---|---|
| Synonyms | (2α,3β)-2,3-Dihydroxyurs-12-en-28-oic acid; 2α-Hydroxyursolic acid; Colosolic acid; Corsolic acid; Glucosol |
| Product Type | Chemical |
| Properties | |
| Formula |
C30H48O4 |
| MW | 472.7 |
| CAS | 4547-24-4 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (10mg/ml) or methanol (1 mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HFGSQOYIOKBQOW-LNGPDNCPSA-N |
| Smiles | CC1CC[C@]2(C(O)=O)CC[C@@]3(C)[C@]4(C)CC[C@@]5([H])C(C)(C)[C@@H](O)C(O)C[C@]5(C)[C@@]4([H])CC=C3[C@]2([H])[C@H]1C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Corosolic acid is a triterpene phytochemical found in medicinal herbal extracts. It possesses antiatherosclerotic, antihyperlipidemic/antidiabetic, antioxidant, anti-inflammatory, antifungal, antiviral and antitumor activities. It is an apoptotic agent that induces caspase-8, -9 and -3 activation and shows antiproliferative effects against cancer cell lines. It stimulates glucose uptake via enhancing insulin receptor phosphorylation and ameliorates obesity in vivo. It has been shown to exhibit antiangiogenic and antilymphangiogenic effects in vitro and in vivo. Has also been shown to inhibit mitochondrial fission and NOX2 expression preventing NLRP3 inflammasome activation. It inhibits adipose tissue inflammation and ameliorate insulin resistance via AMPK activation and is an α-glucosidase inhibitor.
(1) K.S Ahn, et al.; Planta Med. 64, 468 (1998) | (2) T. Miura, et al.; Biol. Pharm. Bull. 29, 585 (2006) | (3) L. Shi, et al.; Eur. J. Pharmacol. 584, 21 (2008) | (4) K. Yamada, et al.; Biol. Pharm. Bull. 31, 651 (2008) | (5) Y. Xu, et al.; Cancer Lett. 284, 229 (2009) | (6) H. Horlad, et al.; Mol. Nutr. Food Res. 57, 1046 (2013) | (7) K.H. Yoo, et al.; Phytother. Res. 29, 714 (2015) | (8) S.J. Kim, et al.; BMB Rep. 49, 276 (2016) | (9) Y. Li, et al.; Antioxid. Redox Signal. 24, 893 (2016) | (10) J. Yang, et al.; Phytomedicine 23, 181 (2016) | (11) M. Ni, et al.; J. Sci. Food Agric. 99, 5881 (2019)





