Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
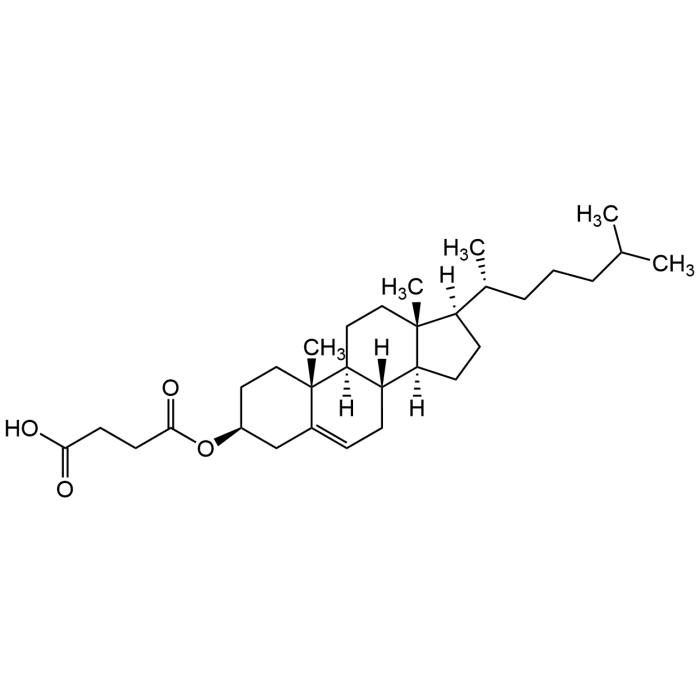
Cholesteryl hemisuccinate
| Product Details | |
|---|---|
| Synonyms | 3β-Hydroxy-5-cholestene 3-hemisuccinate; 5-Cholesten-3β-ol 3-hemisuccinate; Cholesteryl hydrogen succinate |
| Product Type | Chemical |
| Properties | |
| Formula | C31H50O4 |
| MW | 486.73 |
| CAS | 1510-21-0 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in chloroform (10mg/ml), DMSO or ethanol (both 1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | WLNARFZDISHUGS-MIXBDBMTSA-N |
| Smiles | C[C@@]12[C@]3([H])[C@](CC=C1C[C@H](CC2)OC(CCC(O)=O)=O)([H])[C@@]4([H])[C@](CC3)([C@@](CC4)([H])[C@H](C)CCCC(C)C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Cholesteryl hemisuccinate (CHEMS) is a cholesterol ester. It is formed by esterifying cholesterol with succinic acid. The chemical structure comprises a cholesterol molecule attached to a succinic acid moiety. It possesses both hydrophilic (water-attracting) and hydrophobic (water-repelling) properties, making it useful as a surfactant. CHEMS can form lyotropic liquid crystals, which are important in the formation of liposomes and other nanoparticle drug delivery systems. CHEMS acts as an ionizable anionic detergent and is commonly used to stabilize unilamellar vesicles and liposomes. CHEMS is commonly used in the formulation of liposomes and other lipid-based drug delivery systems. These systems can encapsulate drugs, enhancing their stability, bioavailability, and controlled release. It has also been used as an emulsifying agent in various vesicular drug delivery systems for anticancer drugs, antibiotics, and oligonucleotides and to solubilize various proteins including chemokine receptor 1 as well as erythrocyte ghosts. In addition is used in the study of membrane proteins and the biophysical properties of cell membranes. CHEMS has also been described to have anticancer activity.
(1) J.E. Carroll, et al.; Life Sci. 32, 1573 (1983) | (2) Y.G. Skornick, et al.; Cancer 58, 650 (1986) | (3) M.W. Fariss, et al.; Cancer Res. 54, 3346 (1994) | (4) D. Bach, et al.; Chem. Phys. Lipids 76, 123 (1995) | (5) J.B. Massey; Biochim. Biophys. Acta 1415, 193 (1998) | (6) I.M. Hafez & P.R. Cullis; Biochim. Biophys. Acta 1463, 107 (2000) | (7) S. Simoes, et al.; Adv. Drug Deliv. Rev. 56, 947 (2004) | (8) W.X. Ding, et al.; Int. J. Pharm. 300, 38 (2005) | (9) H. Xu, et al.; Drug Dev. Ind. Pharm. 34, 134 (2008) | (10) S.J. Allen, et al.; Protein Expr. Purif. 66, 73 (2009) | (11) W. Kulig, et al.; J. Mol. Model 20, 2121 (2014) | (12) L. Luo, et al.; Colloids Surf. B Biointerfaces 172, 262 (2018) | (13) H. Shah, et al.; Pharmaceutics 14, 129 (2022)





