Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
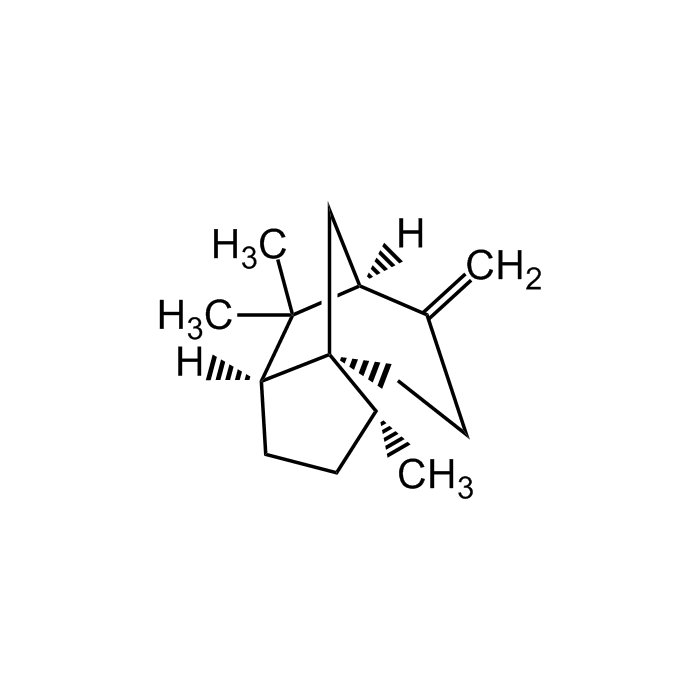
(+)-β-Cedrene
| Product Details | |
|---|---|
| Synonyms | (1S,2R,5S)-8-Methylene-2,6,6-trimethyltricyclo[5.3.1.01.5]undecane |
| Product Type | Chemical |
| Properties | |
| Formula |
C15H24 |
| MW | 204.35 |
| CAS | 546-28-1 |
| Purity Chemicals | ≥95% (sum of enantiomers, GC) |
| Appearance | Colourless to light yellow liquid. |
| Solubility | Soluble in chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | DYLPEFGBWGEFBB-OSFYFWSMSA-N |
| Smiles | C=C1[C@@]2([H])C(C)(C)[C@@](CC[C@H]3C)([H])[C@@]3(CC1)C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Along with α-Cedrene, β-Cedrene is one of two isomers that form Cedrene, a sesquiterpene derived from the essential oils of cedar, also used as a raw material for biofuel development. Like α-Cedrene, β-Cedrene's crisp scents of pine and wood have contributed to its widespread use in fragrances, cosmetics, while its gentle sweet taste has seen it incorporated in beverages, sweeteners and baked goods. Similar to its isomer twin, β-Cedrene is a valued compound in traditional and holistic medicine. Studies have confirmed its therapeutic potency as anti-inflammatory, antiseptic, antispasmodic and antimicrobial agent. β-Cedrene has been found to be a competitive inhibitor of CYP2B6 with Ki value of 1.6µM.
(1) W.J.Kerr, et al.; Org Lett. 3, 2945 (2001) | (2) W.H. Johnston, et al.; Phytother. Res. 15, 586 (2001) | (3) H.U. Jeong, et al.; J. Toxicol. Environ. Health A. 77, 1522 (2014) | (4) H.Y. Zhang, et al.; Molecules 22, 381 (2017) | (5) K.W. Harrison & B.G. Harvey; Sustainable Energy & Fuels 1, 467 (2017)





