Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
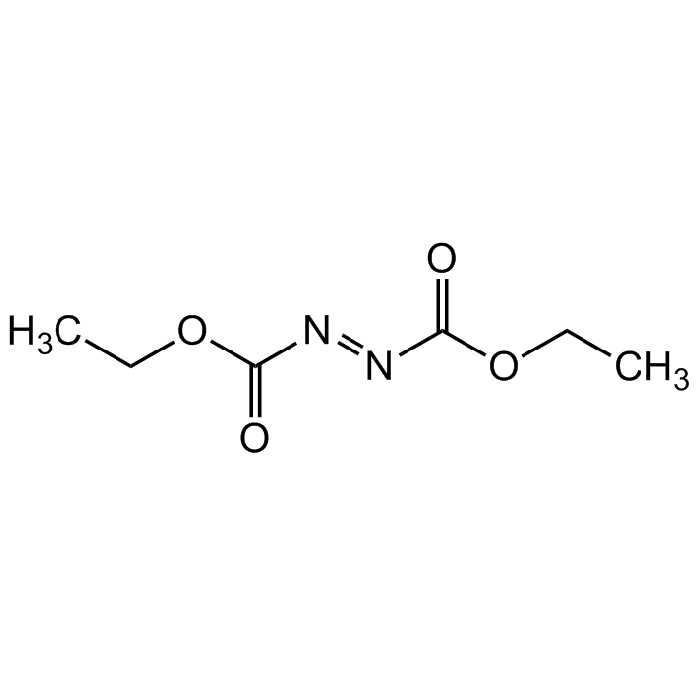
Diethyl azodicarboxylate
| Product Details | |
|---|---|
| Synonyms | DEAD; 1,2-Ethoxycarbonyl diazene; Diethoxycarbonyldiazene; NSC 3474; NSC 679015; Unifoam AZ-AE 200 |
| Product Type | Chemical |
| Properties | |
| Formula |
C6H10N2O4 |
| MW | 174.15 |
| CAS | 1972-28-7 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% |
| Appearance | Red clear liquid. |
| Solubility | Soluble in toluene, chloroform or DCM. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | FAMRKDQNMBBFBR-BQYQJAHWSA-N |
| Smiles | O=C(/N=N/C(OCC)=O)OCC |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Reagent for synthesis. Diethyl azodicarboxylate acts as a dienophile used in Diels-Alder reactions. It is used as a reagent in α-thiocyanation of enolizable ketones with ammonium thiocyanate and annulation of N-protected imines. It acts as a reactant for the preparation of immunostimulants α-Galactosylceramides, bisubstrate inhibitors with molecular recognition at the active site of catechol-O-methyltransferase and aza-beta-lactams via NHC-catalyzed [2 + 2] cycloaddition with ketenes. It is a commonly used activating reagent in Mitsunobu reaction and used in the synthesis of pharmaceuticals like zidovudine and procarbazine. In addition, it is an efficient dehydrogenating agent, which is involved in the preparation of aldehydes, disulfides, hydrazo groups from alcohols, thiols and azo goups, respectively. It is also a good electron acceptor. It is mostly known as a key component of the Mitsunobu reaction, a common strategy for the preparation of an amine, azide, ether, thioether or ester from the corresponding alcohol.
(1) F. Yoneda, et al.; JACS 88, 2328 (1966) | (2) E.C. Taylor & F. Yoneda; Chem. Commun. 1967, 199 (1967) | (3) E.E. Smissman & A. Makriyannis; J. Org. Chem. 38, 1652 (1973) | (4) L.A. Pauette; Encycl. Reag. Org. Synth. 3, 1790 (1995) | (5) M. Shi & G.-L. Zhao; Tetrahedr. 60, 2083 (2004) | (6) R. Dembinski; Eur. J. Org. Chem. 2004, 2763 (2004) | (7) V. Nair, et al.; Angew. Chem. Int. Ed. Engl. 46, 2070 (2007) | (8) E.J. Stoner & A.C. Hart; Encycl. Reag. Org. Synth. (2010)





