Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
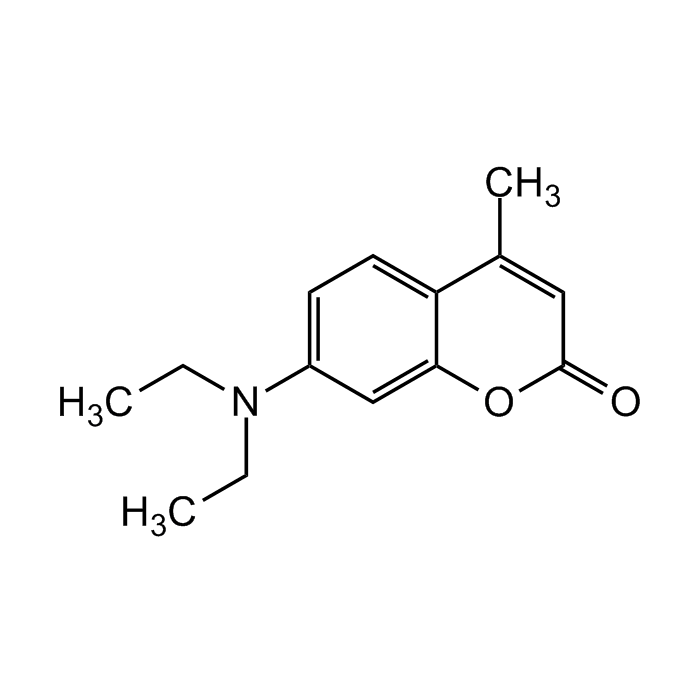
7-Diethylamino-4-methylcoumarin
| Product Details | |
|---|---|
| Synonyms | Coumarin 1; Coumarin 460; Coumarin 47; 7D4MC; C47; C460; DEMC; C.I. 551100; C.I. Fluorescent Brightener 140; C.I. Fluorescent Brightener 52; Hakkol P; Neo-Super HR 1; NSC 61830 |
| Product Type | Chemical |
| Properties | |
| Formula |
C14H17NO2 |
| MW | 2932.29 |
| CAS | 91-44-1 |
| RTECS | GN6370000 |
| Purity Chemicals | ≥99% (Titration) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in methanol or chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AFYCEAFSNDLKSX-UHFFFAOYSA-N |
| Smiles | CC(C1=CC=C(N(CC)CC)C=C1O2)=CC2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
7-Diethylamino-4-methylcoumarin (also called Coumarin 1 or Coumarin 460) is a bichromophoric fluorescent laser dye. It can be used as a laser dye, a luminescent phosphor in LEDs, a luminescent probe and sensor in biological applications, and a fluorescer in rewritable optical drives. The efficient fluorescent property of this compound is utilized to sense the Pd2+ selectively and sensitively.It is also used as a fluorescent whitening agent (FWAs), which is a chemical added to fabrics and paper during manufacture to increase whiteness and brightness. Spectral data: λex: 373nm (ethanol); λmax: 460nm; Laser range: 450-484nm.
(1) K. Kato; IEEE J. Quantum Electron. 11, 373 (1975) | (2) S. Vajda, et al.; J. Chem. Soc. Faraday Trans. 91, 867 (1995) | (3) H.K. Law, et al.; Appl. Opt. 37, 5694 (1998) | (4) M.V. Rusalov, et al.; J. Fluoresc. 14, 193 (2004) | (5) H. Jung, et al.; J. Appl. Toxicol. 32, 654 (2012) | (6) K. Benson, et al.; Adv. Functional Mater. 27, 1702955 (2017) | (7) T. Song, et al.; J. Photochem. Photobiol. B. Biol. 183, 302 (2018) | (8) S. Song, et al.; J. Mater. Chem. C 6, 10704 (2018) | (9) B.C.M.A. Ashwin, et al.; J. Photochem. Photobiol. B 183, 302 (2018) | (10) C. Wang, et al.; Laser Phys. 31, 025801 (2021)





