Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
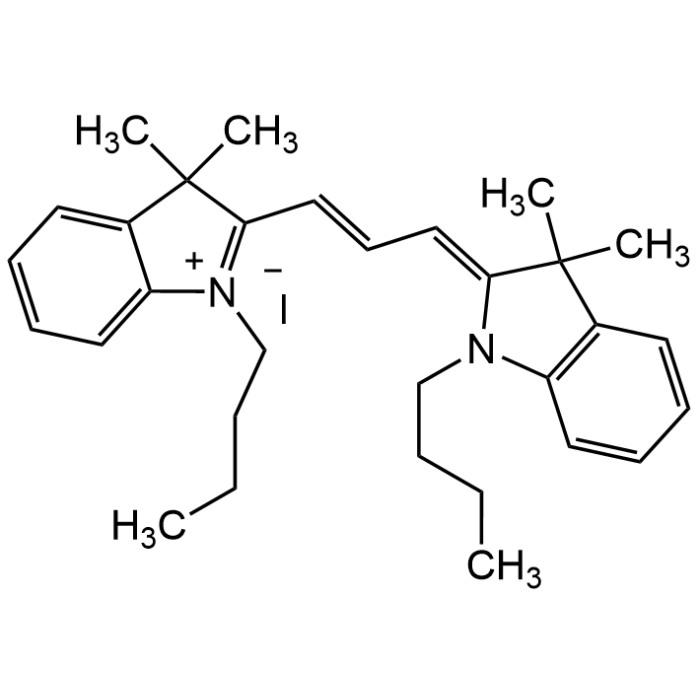
1,1'-Dibutyl-3,3,3',3'-tetramethyl indocarbocyanine iodide
As low as
116
CHF
CHF 116.00
In stock
Only %1 left
CDX-D0224-M05050 mgCHF 116.00
CDX-D0224-M100100 mgCHF 200.00
CDX-D0224-M250250 mgCHF 400.00
| Product Details | |
|---|---|
| Synonyms | DiIC4(3) |
| Product Type | Chemical |
| Properties | |
| Formula | C31H41N2 . I |
| MW | 568.58 |
| CAS | 132752-00-2 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Violett crystalline solid. |
| Solubility | Soluble in DMSO or methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | CVTQMCMIKOZBTI-UHFFFAOYSA-M |
| Smiles | CCCCN\1C2=CC=CC=C2C(/C1=C\C=C\C3=[N+](C4=CC=CC=C4C3(C)C)CCCC)(C)C.[I-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep under inert gas. Protect from light and oxygen. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
DiIC4(3) is a fluorescent carbocyanine dye with butyl chains widely used for membrane staining due to its hydrophobic nature, which helps it intercalate well into lipid bilayers. Carbocyanine dyes are unique organic molecules that contain a conjugated electron-deficient system between two heterocyclic nitrogen atoms that provides characteristically long absorption and emission wavelengths. It can be used for cell tracking as it is stably retained in membranes and in fixed tissue slices for anterograde and retrograde tracing. Spectral Data: Excitation max: ~550-555nm, Emission max: ~565-570nm (accord. Lit). Also used as intermediate for the synthesis of selective protein arginine methyltransferases (PRMT) inhibitors. Intermediate for the synthesis of fluorescent probes.
Product References
(1) G. Suaerwein & G.B. Schuster; J. Phys. Chem. 95, 1903 (1991) | (2) G. Beckford, et al.; Talanta 92, 45 (2012) | (3) S.H. Sinha, et al.; Eur. J. Med. Chem. 54, 647 (2012) | (4) H. Hu, et al.; J. Med. Chem. 58, 1228 (2015) | (5) M. Matsui, et al.; Tetrahedron 71, 3528 (2015)





