Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
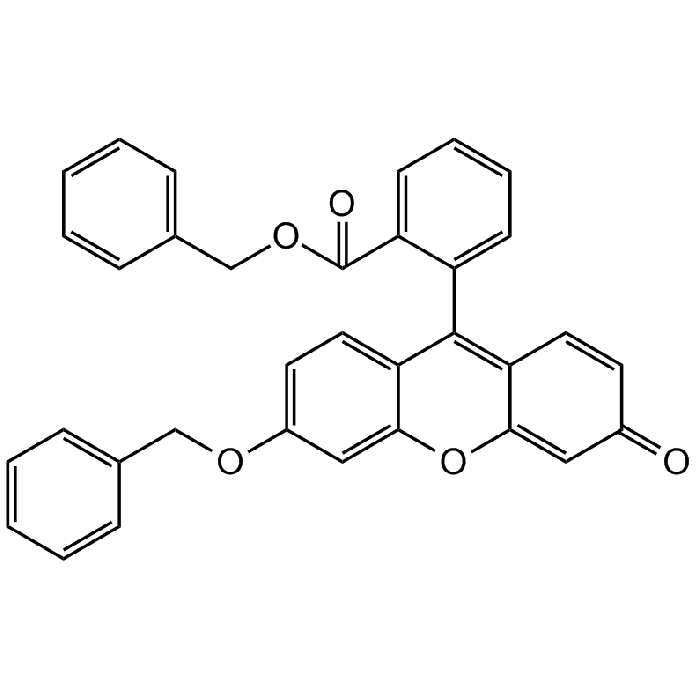
Dibenzylfluorescein
| Product Details | |
|---|---|
| Synonyms | DBF; NSC 645658 |
| Product Type | Chemical |
| Properties | |
| Formula |
C34H24O5 |
| MW | 512.55 |
| CAS | 97744-44-0 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | Faint to dark orange powder. |
| Solubility | Soluble in DMSO (10mg/ml) or DMF (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | YZJGKSLPSGPFEV-UHFFFAOYSA-N |
| Smiles | O=C(OCC1=CC=CC=C1)C2=CC=CC=C2C(C3=CC=C(OCC4=CC=CC=C4)C=C3O5)=C6C5=CC(C=C6)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Dibenzylfluorescein is a fluorogenic probe that acts as a substrate for specific cytochrome P450 (CYP) isoforms, including CYP3A4, CYP2C8, CYP2C9, CYP2C19 and aromatase (CYP19). It is dealkylated by these CYP isoforms to produce fluorescein benzyl ether, which is further hydrolyzed to fluorescein by the addition of base (typically 2 M NaOH). Dibenzylfluorescein is typically used near its apparent Km value of 0.87-1.9 µM. The fluorescence of fluorescein is evaluated using excitation/emission wavelengths of 485/538nm. Dibenzylfluorescein is used to detect changes in CYP catalytic activity caused by drugs or disease.
(1) D.M. Stresser, et al.; Drug Metab. Dispos. 28, 1440 (2000) | (2) M.T. Donato, et al.; Drug Metab. Dispos. 32, 699 (2004) | (3) E.R. Troesken, et al.; Toxicol. 219, 33 (2006) | (4) K.A. Salminen, et al.; Drug Metab. Dispos. 39, 412 (2011) | (5) D. Moutinho, et al.; Drug Metab. Dispos. 40, 754 (2012)





