Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
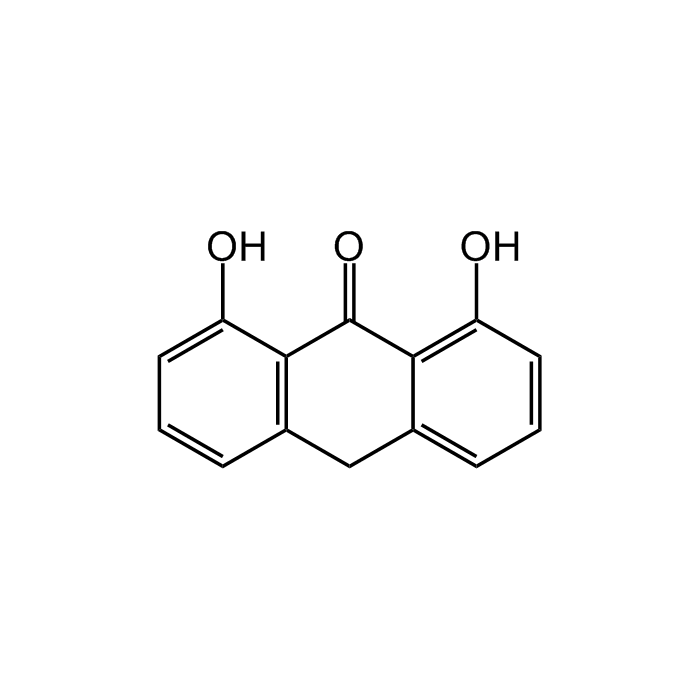
Anthralin
| Product Details | |
|---|---|
| Synonyms | Dithranol; 1,8-Dihydroxyanthrone; Anthracene-1,8,9-triol; NSC 43970; NSC 629313 |
| Product Type | Chemical |
| Properties | |
| Formula | C14H10O3 |
| MW | 226.2 |
| CAS | 1143-38-0 |
| RTECS | CB8927000 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Faint yellow to yellow powder. |
| Solubility | Soluble in acetone. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NUZWLKWWNNJHPT-UHFFFAOYSA-N |
| Smiles | OC1=CC=CC2=C1C(=O)C1=C(O)C=CC=C1C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Anthralin is a leukotriene biosynthesis inhibitor. It inhibits LTB4 ω-oxidation and disrupts mitochondria function. Anthralin is used in the treatment of psoriasis, as a fungicide, in the treatment of ringworm infections and in chronic dermatoses. It accumulates in mitochondria where it interferes with the supply of energy to the cell, probably by the oxidation of dithranol releasing free radicals. This impedes DNA replication and so slows the excessive cell division that occurs in psoriatic plaques. Numerous studies have demonstrated anti-proliferative and anti-inflammatory effects of anthralin on psoriatic and normal skin. It is also used as matrix substance for MALDI-MS (matrix-assisted laser desorption ionization MS).
(1) R.E. Ashton, et al.; J. Am. Acad. Dermatol. 9, 173 (1983) | (2) J.M. Schröder; J. Invest. Dermatol. 87, 624 (1986) | (3) C.E. Orfanos, et al.; Drugs 34, 459 (1987) (Review) | (4) L. Kemeny, et al.; Skin Pharmacol. 3, 1 (1990) | (5) K. Müller; Biochem. Pharmacol. 53, 1215 (1997) (Review) | (6) K. Pavithran; Indian J. Dermatol. Venereol. Leprol. 67, 104 (2001) | (7) C.H. Le, et al.; Anal. Chem. 84, 8391 (2012) | (8) C. Ronpirin & T. Tencomnao; Genet. Mol. Res. 11, 412 (2012) | (9) S.E. George, et al.; J. Pharm. Pharmacol. 65, 552 (2013)