Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
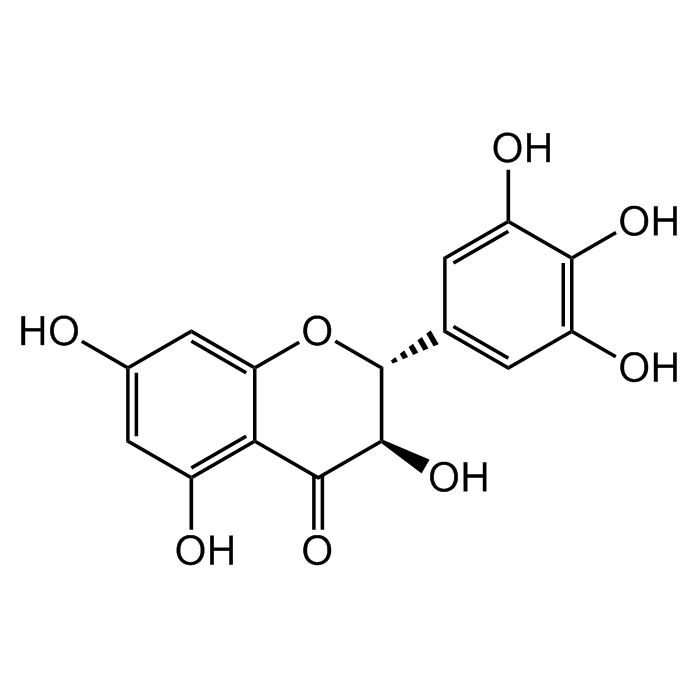
Dihydromyricetin
| Product Details | |
|---|---|
| Synonyms | (+)-Dihydromyricetin; (2R,3R)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxyphenyl)-2,3-dihydrochromen-4-one; 3,3',4',5,5',7-Hexahydroxyflavanone; Ampelopsin; Ampeloptin; DHM |
| Product Type | Chemical |
| Properties | |
| Formula |
C15H12O8 |
| MW | 320.25 |
| CAS | 27200-12-0 |
| RTECS | DJ2982375 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to beige powder. |
| Solubility | Soluble in DMSO (10mg/ml), DMF (10mg/ml) or ethanol (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | KJXSIXMJHKAJOD-LSDHHAIUSA-N |
| Smiles | O[C@H]1C(C2=C(O)C=C(O)C=C2O[C@@H]1C3=CC(O)=C(O)C(O)=C3)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Dihydromyricetin is a flavanonol originally isolated from A. grossedentata. Dihydromyricetin has been demonstrated to show antioxidative, anti-inflammatory, anticancer, antimicrobial, cell death-mediating, neuroprotective and lipid and glucose metabolism-regulatory activities. It also has been found to have anti-alcohol intoxication effects, associated with its action as a positive modulator of GABA-A receptors at the benzodiazepine site. Dihydromyricetin may scavenge ROS to protect against oxidative stress or potentiate ROS generation to counteract cancer cells selectively without any effects on normal cells. Dihydromyricetin may be associated with several different molecules involved in cellular apoptosis, oxidative stress and inflammation, such as AMP-activated protein kinase (AMPK), mitogen-activated protein kinase (MAPK), protein kinase B (Akt), nuclear factor-κB (NF-κ), nuclear factor E2-related factor 2 (Nrf2), ATP-binding cassette transporter A1 (ABCA1) or peroxisome proliferator-activated receptor-γ (PPARγ). It has been reported to effectively modulate growth factor receptor (VEGFR2 and PDGFRβ) mediated signaling, TRAIL/TRAIL-R pathway, JAK/STAT and mTOR-driven signaling in different cancers. It has been shown to stimulate irisin secretion and to inhibit NLRP3 inflammasome-dependent pyroptosis. It also has anti-neuroinflammatory activities in Alzheimer's and Parkinson's diseases and improves conginitve deficits. It acts as a potential preventive or therapeutic agent in treating multiple diseases, such as diabetes mellitus, atherosclerosis, nonalcoholic fatty liver disease and osteoporosis.
(1) Y.S. Zhang, et al.; Yao Xue Xue Bao 38, 241 (2003) | (2) J. Xia, et al.; Food Chem. Toxicol. 66, 7 (2014) | (3) J. Liang, et al.; Neurochem. Res. 39, 1147 (2014) | (4) J. Liang, et al.; Neurochem. Res. 39, 1171 (2014) | (5) J. Liu, et al.; Oncol. Lett. 8, 1645 (2014) | (6) L. Shi, et al.; Mol. Cell Endocrinol. 409, 92 (2015) | (7) S. Chen, et al.; Pharmacol. Res. 99, 74 (2015) | (8) Q. Zhou, et al.; Mol. Cell Endocrinol. 412, 349 (2015) | (9) Z.X. Ren, et al.; Acta Pharmacol. Sin. 37, 1315 (2016) | (10) R. Wang, et al.; Scanning 38, 901 (2016) | (11) B. Xu, et al.; Mol. Med. Rep. 15, 3674 (2017) | (12) H. Li, et al.; Evid. Based Complement Alternat. Med. 2017, 1053617 (2017) (Review) | (13) Q. Hu, et al.; Biofactors 44, 123 (2018) | (14) J. Feng, et al.; CNS Neurosci. Ther. 24, 1207 (2018) | (15) J. Zhang, et al.; Front. Pharmacol. 9, 1204 (2018) (Review) | (16) H. Tong, et al.; Eur. J. Pharmacol. 870, 172888 (2019) (Review) | (17) S. Fayyaz, et al.; Cell Mol. Biol. 65, 15 (2019) (Review)





