Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
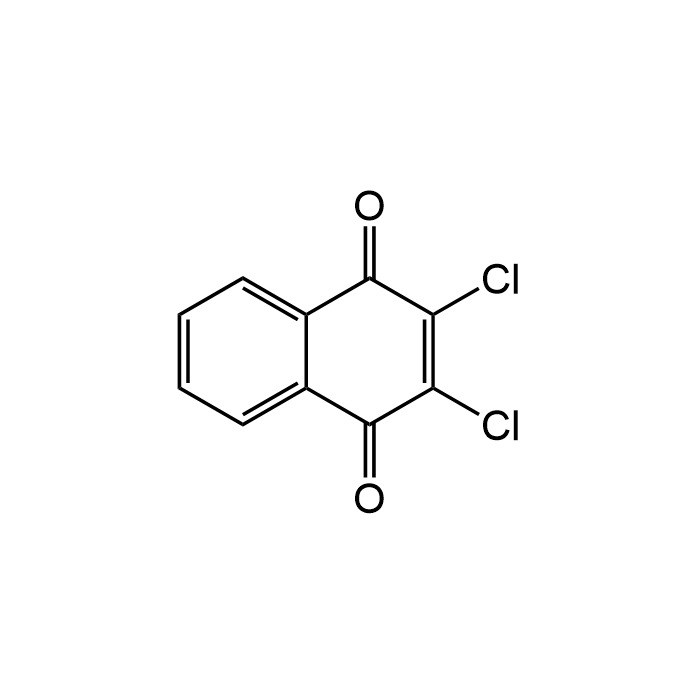
Dichlone
| Product Details | |
|---|---|
| Synonyms | 2,3-Dichloro-1,4-naphthoquinone; CNQ; Dichlon; NSC 537; USR 604 |
| Product Type | Chemical |
| Properties | |
| Formula | C10H4Cl2O2 |
| MW | 227.04 |
| CAS | 117-80-6 |
| RTECS | QL7525000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (GC) |
| Appearance | White to light yellow powder. |
| Solubility | Soluble in chloroform or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | SVPKNMBRVBMTLB-UHFFFAOYSA-N |
| Smiles | ClC1=C(Cl)C(=O)c2ccccc2C1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 6 months after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Dichlone is a naphthoquinone derivative and is widely used in various applications, particularly as a pesticide (fungicide and algicide). This compound can be used as an analytical reference. Dichlone shows several biological properties. It is a potent redox mediator, inducing oxidative stress, has been shown to inhibit ryanodine receptors and acts as a potent DNA methylation inhibitor (EC50=460nM against Dnmt3A/3L catalytic complex). Dichlone is also used as an organic catalyst or as chemical intermediate for synthetic procedures (dyes, pigments) or for spectrophotometric analysis of primary aliphatic amines.
(1) A.A. Abou-Ouf, et al.; J. Pharm. Sci. 62, 1700 (1973) | (2) H.C. Sikka, et al.; Chem. Biol. Interact. 9, 261 (1974) | (3) C.A. Pritsos, et al.; Arch. Biochem. Biophys. 217, 98 (1982) | (4) F.A. El-Yazbi, et al.; J. Pharm. Biomed. Anal. 19, 819 (1999) | (5) C. Morisseau, et al.; Environ Health Perspect. 117, 1867 (2009) | (6) P.P. Vaughan, et al.; Photochem. Photobiol. 86, 1327 (2010) | (7) A. Ceccaldi, et al.; ACS Chem. Biol. 8, 543 (2013) | (8) T.S. Belal, et al.; Spectrochim. Acta A Mol. Biomol. Spectrosc. 155, 47 (2016) | (9) A.A. Petrova, et al.; FEBS Lett. 592, 2220 (2018)





