Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
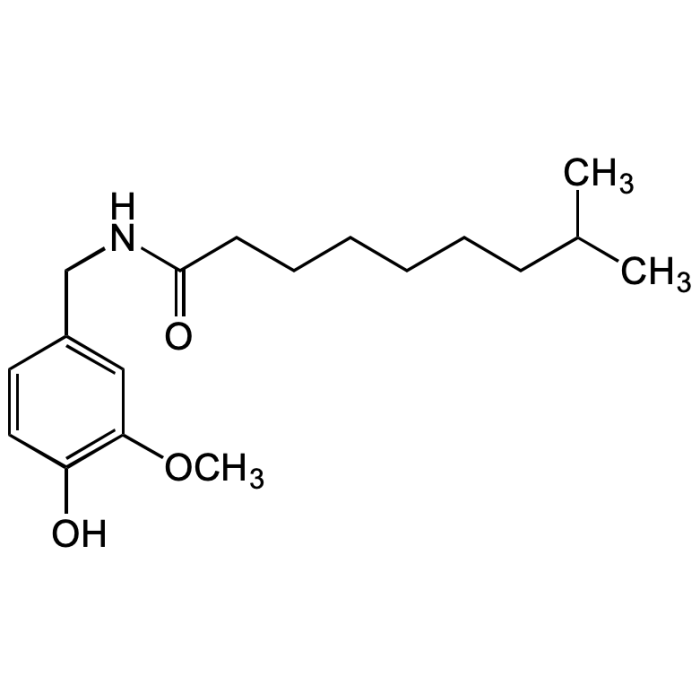
Dihydrocapsaicin
| Product Details | |
|---|---|
| Synonyms | 6,7-Dihydrocapsaicin; 8-Methyl-N-vanillylnonanamide; N-[(4-Hydroxy-3-methoxyphenyl)methyl]-8-methylnonenamide |
| Product Type | Chemical |
| Properties | |
| Formula | C18H29NO3 |
| MW | 307.43 |
| CAS | 19408-84-5 |
| RTECS | RA5998000 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in ethanol, DMSO or DMF (30mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | XJQPQKLURWNAAH-UHFFFAOYSA-N |
| Smiles | OC1=C(OC)C=C(CNC(CCCCCCC(C)C)=O)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Dihydrocapsaicin is a capsaicinoid and analog and congener of capsaicin in chili peppers (capsicum). Dihydrocapsaicin accounts for about 10% of the total capsaicinoid mixture and separation by HPLC is required in order to obtain pure dihydrocapsaicin. Dihydrocapsaicin, similar as capsaicin elicits a sensation of burning pain by activation of VR1 on small, non-myelinated polymodal C-type nociceptive nerve fibers. VR1 (vanilloid receptor 1) is a heat activated calcium ion channel which functions as a part of the normal nociceptive pain pathway. Shown to be analgesic against arthritis pain and inflammation and protective effects against high cholesterol levels and obesity. Induced autophagy, apoptosis in glioma cells via ROS-dependent pathways.
(1) E. Leete & W.C. Louden; JACS 90, 6837 (1968) | (2) M.S. Miller, et al.; Eur. J. Pharmacol. 83, 289 (1982) | (3) P.M. Gannett, et al.; J. Org. Chem. 53, 1064 (1988) | (4) M.J. Caterina, et al.; Nature 389, 816 (1997) | (5) J. Szocsany; Neuropeptides 38, 377 (2004) | (6) R. Kempaiah, et al.; Mol. Cell. Biol. 275, 7 (2005) | (7) S.H. Oh, et al.; Autophagy 4, 1009 (2008) | (8) M. Halme, et al.; J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1009-1010, 17 (2016) | (9) J.J. Zhao, et al.; Arch. Biochem. Biophys. 604, 27 (2016) | (10) L. Xie, et al.; Mol. Med. Rep. 14, 4198 (2016)





