Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
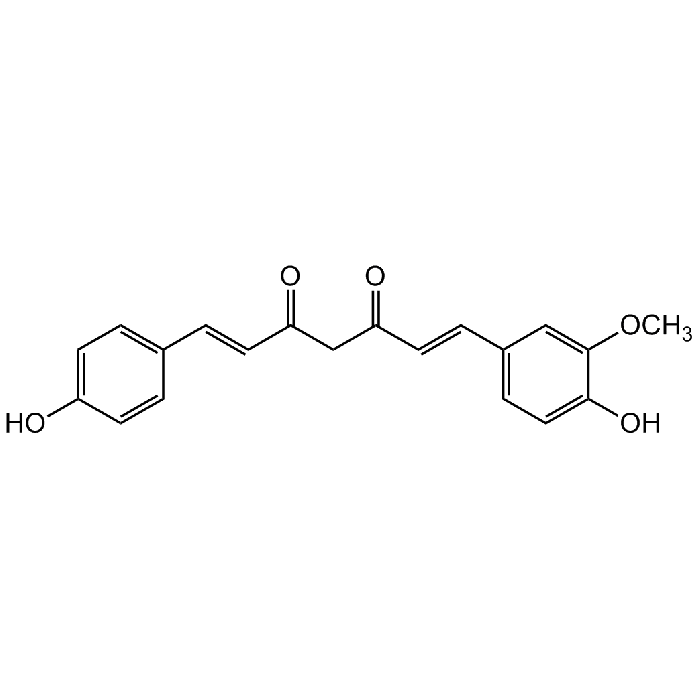
Demethoxycurcumin
| Product Details | |
|---|---|
| Synonyms | DMC; (E,E)-1-(4-Hydroxy-3-methoxyphenyl)-7-(4-hydroxyphenyl)-1,6-heptadiene-3,5-dione; Desmethoxycurcumin; Monodemethoxycurcumin |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H18O5 |
| MW | 338.35 |
| CAS | 22608-11-3 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow to orange powder. |
| Solubility | Soluble in DMSO (10mg/ml) or DMF (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HJTVQHVGMGKONQ-LUZURFALSA-N |
| Smiles | OC1=CC=C(/C=C/C(CC(/C=C/C2=CC=C(O)C(OC)=C2)=O)=O)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Demethoxycurcumin (DMC) is a natural demethoxy derivative of curcumin with anti-inflammatory and anti-cancer properties. DMC suppresses cell proliferation, migration and invasion in cancer cells. It down-regulates the transcriptional coactivator p300, suppressing the Wnt/β-catenin pathway, and inhibits lipopolysaccharide induction of iNOS by blocking NF-κ activation. It also has been shown to inhibit energy metabolic and oncogenic signaling pathways through AMPK activation. In addition it is a potent inhibitor of P-Type ATPases. Shown to have neuroprotective properties. It also has anti-plasmodial activity.
(1) S.K. Sandur, et al.; Carcinogenesis 28, 1765 (2007) | (2) L.Y. Guo, et al.; Arch. Pharm. Res. 31, 490 (2008) | (3) M.J. Ryu, et al.; BBRC 377, 1304 (2008) | (4) L. Zhang, et al.; Int. Immunopharmacol. 10, 331 (2010) | (5) Y.L. Liu, et al.; Mol. Med. Rep. 4, 675 (2011) | (6) X. Ni, et al.; Oncol. Rep. 28, 85 (2012) | (7) O.B. Villaflores, et al.; Taiwan. J. Obstet. Gynecol. 51, 554 (2012) | (8) J.M. Shieh, et al.; J. Agric. Food Chem. 61, 6366 (2013) | (9) R. Munigunti, et al.; Nat. Prod. Res. 28, 359 (2014) | (10) T.T. Dao, et al.; PLoS One 11, e0163260 (2016) | (11) M. Ramkumar, et al.; BMC Complement Altern. Med. 17, 217 (2017) | (12) C.C. Lin, et al.; Anticancer Res. 38, 2761 (2018) | (13) M. Hatamipour, et al.; J. Cell Physiol. 233, 9247 (2018) | (14) M. Hatamipour, et al.; J. Cell Physiol. 234, 19320 (2019)





