Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
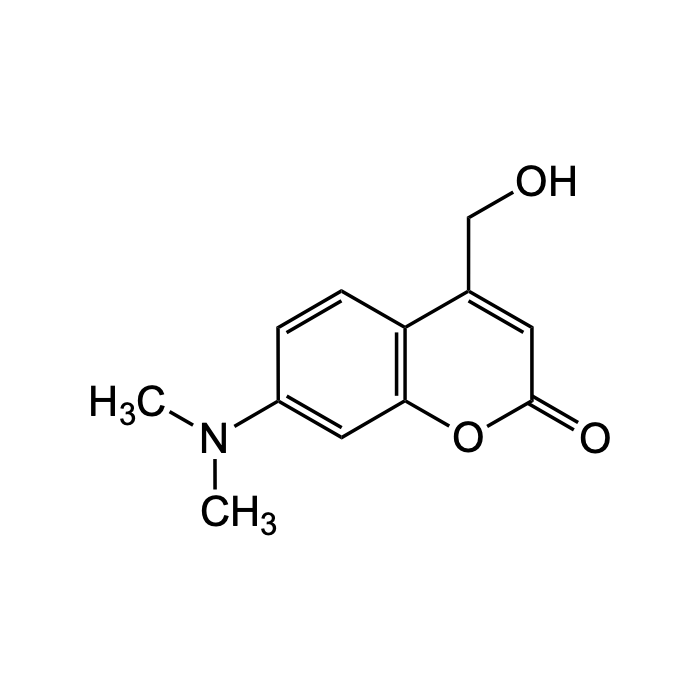
7-(Diethylamino)-4-(hydroxymethyl)coumarin
| Product Details | |
|---|---|
| Synonyms | 7-(diethylamino)-4-(hydroxymethyl)-2H-chromen-2-one; DEACM; DEACM-OH |
| Product Type | Chemical |
| Properties | |
| Formula | C14H17NO3 |
| MW | 247.29 |
| CAS | 54711-38-5 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (GC) |
| Appearance | White to light brown powder. |
| Solubility | Soluble in chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NMZSXNOCNJMJQT-UHFFFAOYSA-N |
| Smiles | CCN(CC)C1=CC2=C(C=C1)C(=CC(=O)O2)CO |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
7-(Diethylamino)-4-(hydroxymethyl)coumarin (DEACM) is a photolabile compound that emits fluorescence upon irradiation and is used in analytical chemistry as a fluorescent probe. The photocleavable nature of DEACM facilitates its use as a photolabile protecting group for "Caged" bioactive molecules. The "caging" of bioactive molecule with photolabile protecting groups in particular has proven to be a useful tool in biochemical research. Caged compounds in which bioactive substances are inactivated with photolabile protecting groups and can be activated by UV or visible light photoirradiation. DEACM has been successfully applied for caging a wide variety of functional groups. It also has been used as a near infrared (NIR) light responsive chromophore.
(1) A.V. Pinheiro, et al.; Nucl. Acids Res. 36, e90 (2008) | (2) B.N. Goguen, et al.; JACS 133, 11038 (2011) | (3) Y. Liang, et al.; Biomaterials 100, 76 (2016) | (4) J. Font, et al.; eLife 6, e23545 (2017) | (5) J. Ma, et al.; Molecules 25, 5325 (2020) | (6) H.-M. Hung, et al.; ACS Chem. Biol. 17, 11 (2022)





