Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
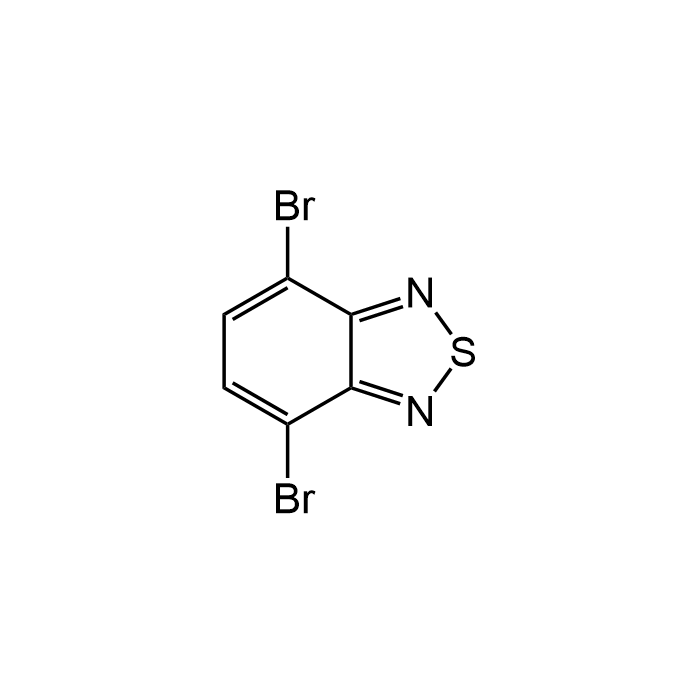
4,7-Dibromobenzo[c]-1,2,5-thiadiazole
As low as
116
CHF
CHF 116.00
In stock
Only %1 left
CDX-D1026-G0055 gCHF 116.00
| Product Details | |
|---|---|
| Synonyms | Dibromo BTD |
| Product Type | Chemical |
| Properties | |
| Formula | C6H2Br2N2S |
| MW | 293.97 |
| CAS | 15155-41-6 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (GC) |
| Appearance | White to yellow to red powder or crystal. |
| Solubility | Soluble in chloroform or DMSO (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | FEOWHLLJXAECMU-UHFFFAOYSA-N |
| Smiles | BrC1=CC=C(Br)C2=NSN=C21 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep under inert gas. Protect from light and oxygen. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
4,7-Dibromobenzo[c]-1,2,5-thiadiazole is the building block or monomer for the synthesis of light-emitting diodes and conducting polymers for organic electronics. It has been used as bulding block for photovoltaic device polymers and organic semiconductors. It is used as an intermediate for the synthesis of poly[N-9'-heptadecanyl-2,7-carbazole-alt-5,5-(4',7'-di-2-thienyl-2',1',3'-benzothiadiazole)] (PCDTBT) and poly[2,1,3-benzothiadiazole-4,7-diyl[4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b:3,4-b']dithiophene-2,6-diyl]] (PCPDTBT). 4,7-Dibromobenzo[c]-1,2,5-thiadiazole functions as an electron-accepting material in organic electronic devices. It acts as a p-type semiconductor, facilitating the transport of positive charge carriers within the device and exhibits high electron mobility and good stability, making it suitable for use in organic field-effect transistors and organic photovoltaic devices.
Product References
(1) J. Kim, et al.; Polymer 51, 390 (2010) | (2) Z.J. Wang, et al.; ChemSusChem 8, 3459 (2015) | (3) M.S. Pavan, et al.; J. Phys. Chem. B 119, 11382 (2015) | (4) F. Lomneck, et al.; Macromol. Rapid Commun. 36, 231 (2015) | (5) S.A.A. Shah, et al.; J. Mat. Sci. Mat. Electr. 27, 4501 (2016) | (6) E.A. Knyazeva & O.A. Rakitin; Chem. Heterocyc. Comp. 53, 855 (2017) | (7) Y. Uozumi & T. Sato; Synfacts 13, 1101 (2017) | (8) A. Sarasola, et al.; JACS 140, 15631 (2018) | (9) Y. Yu, et al.; ACS Omega 3, 16347 (2018)





