Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
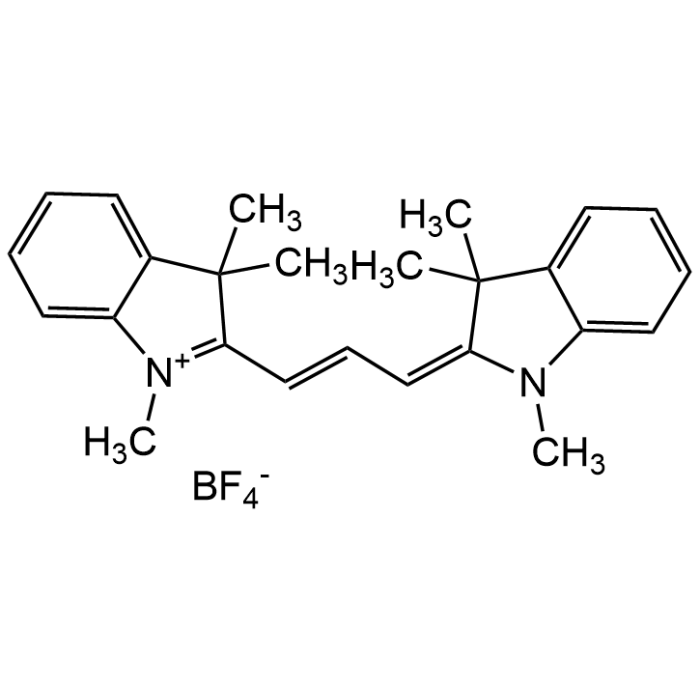
1,1'-Dimethyl-3,3,3',3'-tetramethyl indocarbocyanine tetrafluoroborate
As low as
161
CHF
CHF 161.00
In stock
Only %1 left
CDX-D1107-M05050 mgCHF 161.00
CDX-D1107-M100100 mgCHF 271.00
CDX-D1107-M250250 mgCHF 561.00
| Product Details | |
|---|---|
| Synonyms | DiIC1(3); HIC; 1,3,3,1',3',3'-Hexamethylindocarbocyanine tetrafluoroborate |
| Product Type | Chemical |
| Properties | |
| Formula | C25H29N2 . BF4 |
| MW | 444.32 |
| CAS | 61575-72-2 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Purple crystalline solid. |
| Solubility | Soluble in DMSO or ethanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QXPZINFOKFHGAQ-UHFFFAOYSA-N |
| Smiles | [B-](F)(F)(F)F.CC1(C2=CC=CC=C2[N+](=C1/C=C/C=C\3/C(C4=CC=CC=C4N3C)(C)C)C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep under inert gas. Protect from light and oxygen. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
DiIC1(3) is a fluorescent carbocyanine dye used mainly for assessing membrane potential in mitochondria and plasma membranes, especially in flow cytometry and fluorescence microscopy. Can also be used as a laser dye. DiIC1(3) has short alkyl chains (methyl groups), so it diffuses more easily but is less stably anchored in membranes than longer-chain versions like DiIC12(3) or DiIC18(3). It is ideal for dynamic membrane potential assessments, not long-term labeling. The dye can be used as a non-reactive fluorophore, for control experiments and for calibration. The BF4 anion is better for organic solvents. Spectral Data: Excitation max: ~ 545nm, Emission max: ~565nm (EtOH).
Product References
(1) A.S. Tatikolov, et al.; Chem. Phys. Lett. 190, 291 (1992) | (2) N.A. Davidenko, et al.; Theoret. Exp. Chem. 34, 158 (1998) | (3) N.A. Davidenko, et al.; Phys. Solid State 41, 179 (1999) | (4) N.K. Ibrayev, et al.; J. Luminesc. 90, 81 (2000) | (5) R.A. Ganeev, et al.; Appl. Phys. B 76, 683 (2003) | (6) N.A. Davidenko, et al.; Spectrochim. Acta Part A 61, 213 (2005) | (7) V.A. Svetlichnyi, et al.; Quantum Electronics 37, 118 (2007) | (8) A.R. Shabelko, et al.; Spectrochim. Acta Part A 321, 124728 (2024)





