Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Emodin
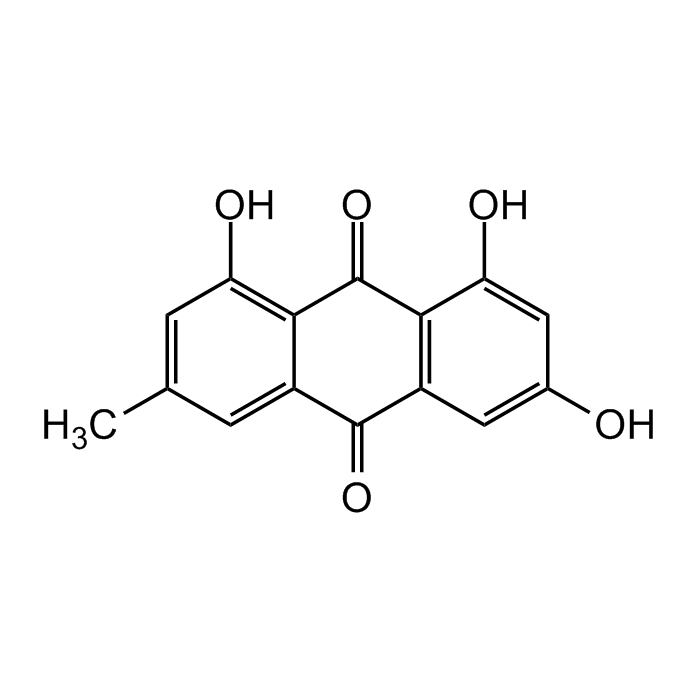
| Product Details | |
|---|---|
| Synonyms | Emodol; Frangulic acid; Archin; NSC 622947; NSC 408120; 1,3,8-Trihydroxy-6-methylanthraquinone; Schuttgelb |
| Product Type | Chemical |
| Properties | |
| Formula |
C15H10O5 |
| MW | 270.24 |
| CAS | 518-82-1 |
| RTECS | CB7920600 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | Yellow powder. |
| Solubility | Soluble in DMSO (10mg/ml) or 100% ethanol. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | RHMXXJGYXNZAPX-UHFFFAOYSA-N |
| Smiles | OC1=C2C(C(C(C=C(O)C=C3O)=C3C2=O)=O)=CC(C)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Emodin is a naturally-occurring anthraquinone found in a variety of plants used in traditional Chinese medicine. It displays anti-inflammatory, antitumor, neuroprotective, antioxidant and anti-diabetic properties. At a molecular level, Emodin inhibits casein kinase II, p56lck, NF-κB, 11β-HSD1, Estrogen receptor ERα and ERβ, androgen receptor (AR) nuclear translocation, VEGFR2, FGFR-1, EGFR, PDGFR-β, Kit and JAK2. It has also been described to inhibit the inflammasome, being a AMPK activator and PPARγ agonist and a P2X7 receptor antagonist.
(1) Vasorelaxant effect of emodin, an anthraquinone from a Chinese herb: H.C. Huang, et al.; Eur. J. Pharmacol. 205, 289 (1991) | (2) Emodin, a protein tyrosine kinase inhibitor from Polygonum cuspidatum: H. Jayasuriya, et al.; J. Nat. Prod. 55, 696 (1992) | (3) Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells: A. Kumar, et al.; Oncogene 17, 913 (1998) | (4) Emodin, an anthraquinone derivative isolated from the rhizomes of Rheum palmatum, selectively inhibits the activity of casein kinase II as a competitive inhibitor: H. Yim, et al.; Planta Med. 65, 9 (1999) | (5) Phytoestrogens from the roots of Polygonum cuspidatum (Polygonaceae): structure-requirement of hydroxyanthraquinones for estrogenic activity: H. Matsuda, et al.; Bioorg. Med. Chem. Lett. 11, 1839 (2001) | (6) Emodin down-regulates androgen receptor and inhibits prostate cancer cell growth: T.L. Cha, et al.; Cancer Res. 65, 2287 (2005) | (7) Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor: A. Muto, et al.; Mol. Cancer Ther. 6, 987 (2007) | (8) Screening of Kit inhibitors: suppression of Kit signaling and melanogenesis by emodin: S.J. Lee, et al.; Phytother. Res. 24, 308 (2010) | (9) Neuroprotective effects of emodin in rat cortical neurons against beta-amyloid-induced neurotoxicity: T. Liu, et al.; Brain Res. 1347, 149 (2010) | (10) Inhibition of ATP-induced macrophage death by emodin via antagonizing P2X7 receptor: L. Liu, et al.; Eur. J. Pharmacol. 640, 15 (2010) | (11) Emodin, a natural product, selectively inhibits 11beta-hydroxysteroid dehydrogenase type 1 and ameliorates metabolic disorder in diet-induced obese mice: Y. Feng, et al.; Br. J. Pharmacol. 161, 113 (2010) | (12) Promotion of adiponectin multimerization by emodin: a novel AMPK activator with PPARγ-agonist activity: Z. Chen, et al.; J. Cell Biochem. 113, 3547 (2012) | (13) The distinct mechanisms of the antitumor activity of emodin in different types of cancer: W.T. Wei, et al.; Oncol. Rep. 30, 2555 (2013) (Review) | (14) Emodin suppresses Wnt signaling in human colorectal cancer cells SW480 and SW620: T. Pooja & D. Karunagaran; Eur. J. Pharmacol. 742, 55 (2014) | (15) Anti-Inflammatory effect of emodin via attenuation of NLRP3 inflammasome activation: J.W. Han, et al.; Int. J. Mol. Sci. 16, 8102 (2015)





