Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
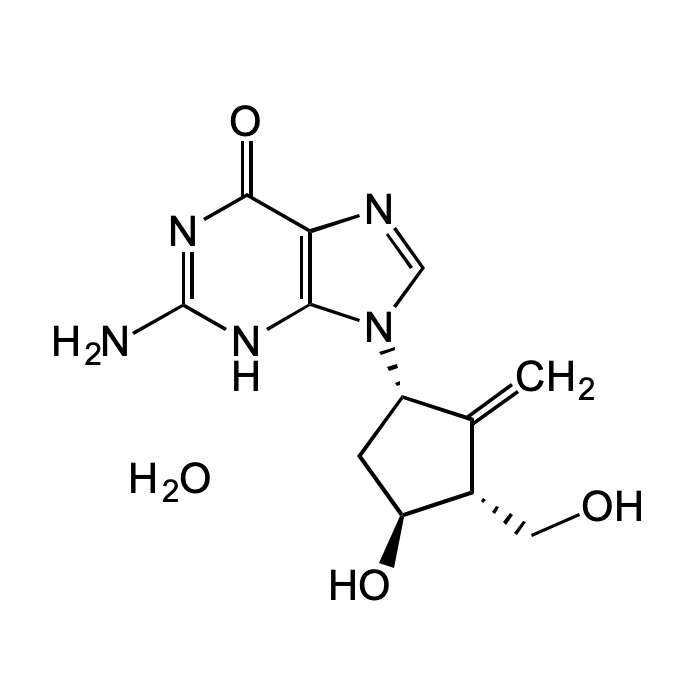
Entecavir monohydrate
| Product Details | |
|---|---|
| Synonyms | Baraclude; BMS 200475; SQ 34,676; 2-Amino-1,9-dihydro-9-[(1S,3R,4S)-4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H-purin-6-one monohydrate |
| Product Type | Chemical |
| Properties | |
| Formula | C12H15N5O3 . H2O |
| MW | 295.29 |
| CAS | 209216-23-9 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Solube in DMSO (10mg/ml), DMF (10mg/ml) or methanol. Sparingly soluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | YXPVEXCTPGULBZ-WQYNNSOESA-N |
| Smiles | O=C1C2=C(N([C@H]3C[C@H](O)[C@@H](CO)C3=C)C=N2)NC(N)=N1.O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Entecavir is an oral antiviral drug used in the treatment of hepatitis B (HBV) infection. It is marketed under the trade name Baraclude (BMS). Entecavir is a potent deoxyguanosine nucleoside analog with antiviral activity selective for hepadnaviruses. In vitro, the active intracellular form of entecavir, entecavir triphosphate, demonstrates a higher binding affinity for HBV DNA polymerase than the natural guanosine triphosphate substrate and effectively inhibits HBV DNA replication at 3 stages in the replication pathway: priming, reverse transcription and DNA-dependent DNA synthesis. Can be also used as a reference compound.
(1) S.F. Innaimo, et al.; Antimicrob. Agents Chemother. 41, 1444 (1997) | (2) M. Seifer, et al.; Antimicrob. Agents Chemother. 42, 3200 (1998) | (3) P. Honkoop & R.A. De Man; Expert. Opin. Investig. Drugs 12, 683 (2003) (Review) | (4) S.J. Matthews; Clin. Ther. 28, 184 (2006) (Review) | (5) K.A. Sims & A.M. Woodland; Pharmacotherapy 26, 1745 (2006) (Review) | (6) P.N. Cheng & T.T. Chang; Expert Rev. Anti. Infect. Ther. 6, 569 (2008) (Review)





