Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
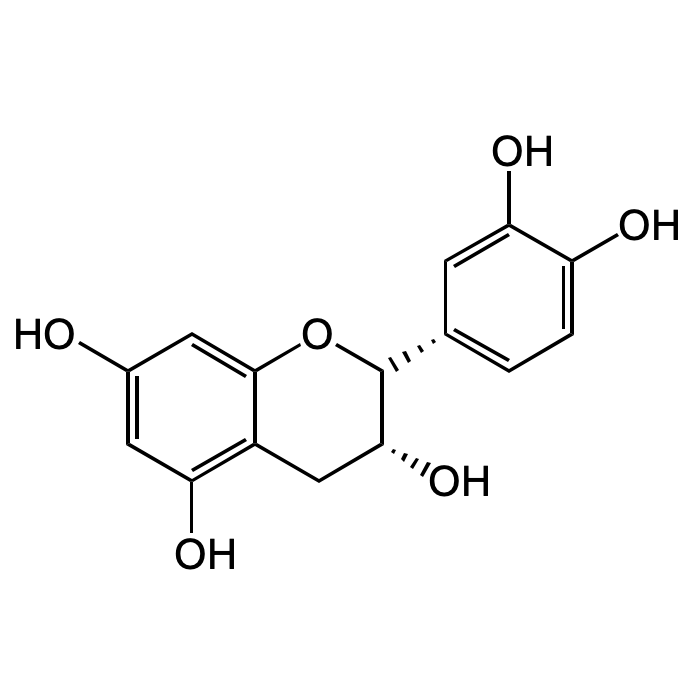
(-)-Epicatechin
| Product Details | |
|---|---|
| Synonyms | EC; epi-Catechin; NSC81161; (-)-cis-3,3',4',5,7-Pentahydroxyflavane, (2R,3R)-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol |
| Product Type | Chemical |
| Properties | |
| Formula | C15H14O6 |
| MW | 290.27 |
| CAS | 490-46-0 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in acetone:water (1:1, 10 mg/ml), DMSO (10 mg/ml), methanol or DMF (~12.5 mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PFTAWBLQPZVEMU-UKRRQHHQSA-N |
| Smiles | OC1=CC(O)=C(C[C@@H](O)[C@@H](C2=CC=C(O)C(O)=C2)O3)C3=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Epicatechin is a natural flavonoid found in green tea. It has been reported to possess an immense antioxidant effect which contributes to its therapeutic effect in diabetes and cancer conditions. The consumption of epicatechin has been shown to reduce blood glucose levels in diabetic patients, while its anticancer effect was attributed to its antioxidant properties, antiangiogenic and direct cytotoxicity to cancer cells. The activity of Epicatechin includes the ability to interact with and neutralize reactive oxygen species (ROS), and to modulate cell signaling including the MAP kinase pathway, which is involved in cell proliferation.
(1) F. Bonilla, et al.; Food Chem. 66, 209 (1999) | (2) M. Suganuma, et al.; Cancer Res. 59, 44 (1999) | (3) J. Terao; J. Med. Invest. 46, 159 (1999) (Review) | (4) J.Z. Xu, et al.; Br. J. Nutr. 91, 873 (2004) | (5) M.H. Ravindranath, et al.; Evidence-Based Compl. Altern. Med. 6, 523 (2009) | (6) C.G. Fraga & P.I. Oteiza; Free Radic. Biol. Med. 51, 813 (2011) (Review) | (7) J. Shay, et al.; Oxid. Med. Cell Longev. 2015, 181260 (2015) (Review) | (8) L.A. Abdulkhaleq, et al.; Vet. World 10, 869 (2017) (Review)





