Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
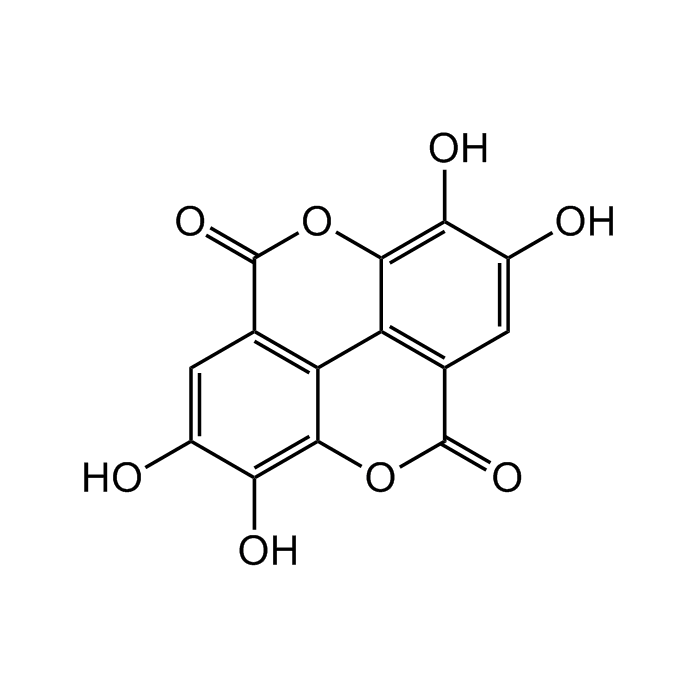
Ellagic acid
| Product Details | |
|---|---|
| Synonyms | 4,4',5,5',6,6'-Hexahydroxydiphenic acid 2,6,2',6'-dilactone; Alizarin Yellow; Benzoaric acid; Elagostasine; Gallogen; Lagistase; TBBD |
| Product Type | Chemical |
| Properties | |
| Formula |
C14H6O8 |
| MW | 302.19 |
| CAS | 476-66-4 |
| RTECS | DJ2620000 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Brown powder. |
| Solubility | Slightly soluble in ethanol or DMSO (<1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AFSDNFLWKVMVRB-UHFFFAOYSA-N |
| Smiles | OC1=CC2=C3C(OC(C4=C3C(OC2=O)=C(O)C(O)=C4)=O)=C1O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Ellagic acid is a polyphenolic antioxidant that is abundant in many fruits, vegetables, plant bark and peels. It has antioxidant, anticancer, neuroprotective, antimutagenic, anti-inflammatory and organ-preserving properties. It alters cytochrome P450 activity and improvea metabolism and clearance of xenobiotics, as well as altera immune function. Selective, ATP-competitive inhibitor of casein kinase 2 (CK2). Ellagic acid also blocks methylation of arginine 17 of histone 3 (H3R17) by coactivator-associated arginine methyltransferase 1 (CARM1) without significantly altering histone acetylase or DNA methyltransferase activity. It acts as a scavenger of oxygen species produced by hydrogen peroxide treatment and as a protector of the DNA double helix from alkylating agent injury. Also reported to inhibit topoisomerases I and II and gyrase and glutathione S-transferase.
(1) C.T. Allen, et al.; Immunopharmacol. Immunotoxicol. 25, 409 (2003) | (2) G. Cozza, et al.; J. Med. Chem. 49, 2363 (2006) | (3) D.H. Han, et al.; Anticancer Res. 26, 3601 (2006) | (4) R. Hayeshi, et al.; Food Chem. Toxicol. 45, 286 (2007) | (5) D. Heber; Cancer Lett. 269, 262 (2008) | (6) C. Bell & S. Hawthorne; J. Pharm. Pharmacol. 60, 139 (2008) (Review) | (7) B.R. Selvi, et al.; J. Biol. Chem. 285, 7143 (2010) | (8) J. Paluszczak, et al.; Toxicol. Lett. 192, 119 (2010) | (9) M.A. Lea, et al.; Anticancer Res. 30, 311 (2010) | (10) H.M. Zhang, et al.; Cancer Biol. Med. 11, 92 (2014) (Review) | (11) W.R. García-Nino & C. Zazueta; Pharmacol. Res. 97, 84 (2015) (Review) | (12) T. Ahmed, et al.; Curr. Pharm. Des. 22, 1350 (2016) (Review) | (13) G. Derosa, et al.; Adv. Exp. Med. Biol. 928, 4473 (Review) | (14) A. Zeb; Mol. Cell Biochem. 448, 27 (2018) (Review) | (15) C. Ceci, et al.; Nutrients 10, E1756 (2018) | (16) S. Alfei, et al.; Eur. J. Med. Chem. 183, 111724 (2019) (Review)





