Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
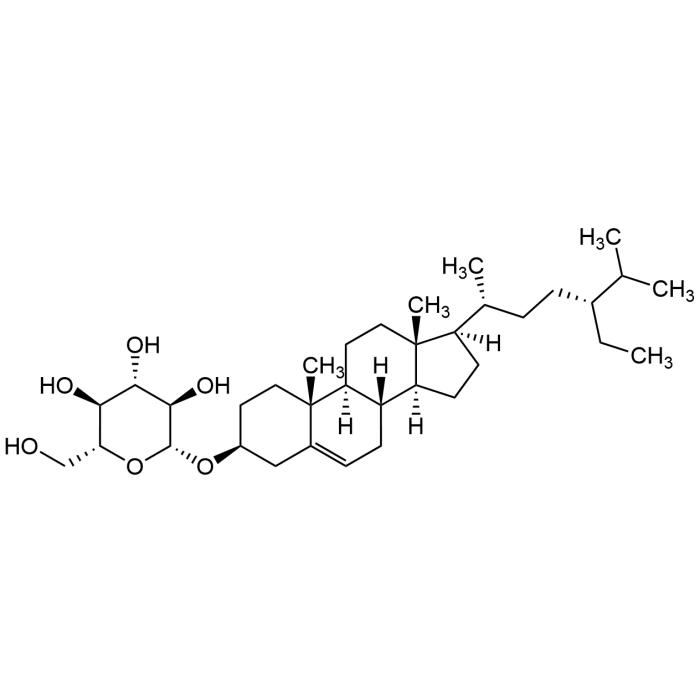
Eleutheroside A
As low as
193
CHF
CHF 193.00
In stock
Only %1 left
CDX-E0233-M01010 mgCHF 193.00
CDX-E0233-M02525 mgCHF 348.00
| Product Details | |
|---|---|
| Synonyms | Daucosterol; Daucosterin; β-Sitosterol β-D-glucoside; β-Sitosterol 3-O-glucoside; β-Sitosterol D-glucopyranoside; NSC 165962 |
| Product Type | Chemical |
| Properties | |
| Formula | C35H60O6 |
| MW | 576.85 |
| CAS | 474-58-8 |
| Source/Host Chemicals | Semisynthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Off-white to pale beige powder. |
| Solubility | Soluble in DMSO (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NPJICTMALKLTFW-OFUAXYCQSA-N |
| Smiles | C[C@@]12[C@](CC[C@]2([H])[C@H](C)CC[C@@H](CC)C(C)C)([H])[C@@]3([H])[C@@](CC1)([H])[C@@]4(C(C[C@H](CC4)O[C@@H]5O[C@@H]([C@H]([C@@H]([C@H]5O)O)O)CO)=CC3)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Eleutheroside A is a natural phytosterol glycoside that exerts multiple bioactivities, like anticancer, anti-inflammatory, immunomodulatory, antibacterial and antioxidant, via modulation of cell signaling, oxidative stress, and immune responses. It modulates immune, metabolic and tumor-associated pathways. Eleutheroside A can inhibit the PI3K/AKT1/mTOR signaling pathway in myeloid-derived suppressor cells (MDSCs), thereby reducing their glycolytic activity and immunosuppressive function in the tumor microenvironment of gastric cancer, which leads to enhanced CD4+/CD8+ T-cell infiltration and slowed tumor growth in vivo.
Product References
(1) J.-H. Lee, et al.; Vaccine 25, 3834 (2007) | (2) E.J. Cho, et al.; Hort. Environ. Biotechnol. 53, 561 (2012) | (3) C. Zhao, et al.; Life Sci. 137, 37 (2015) | (4) L.H. Jiang, et al.; J. Steroid Biochem. Mol. Biol. 152, 45 (2015) | (5) J. Zeng, et al.; Molecules 22, 862 (2017) | (6) H. Dehghan, et al.; Ind. Crops Prod. 117, 303 (2018) | (7) P. Gao, et al.; Biosci. Trends 13, 160 (2019) | (8) X. Jiang, et al.; Int. Immunopharmacol. 159, 114907 (2025)





