Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
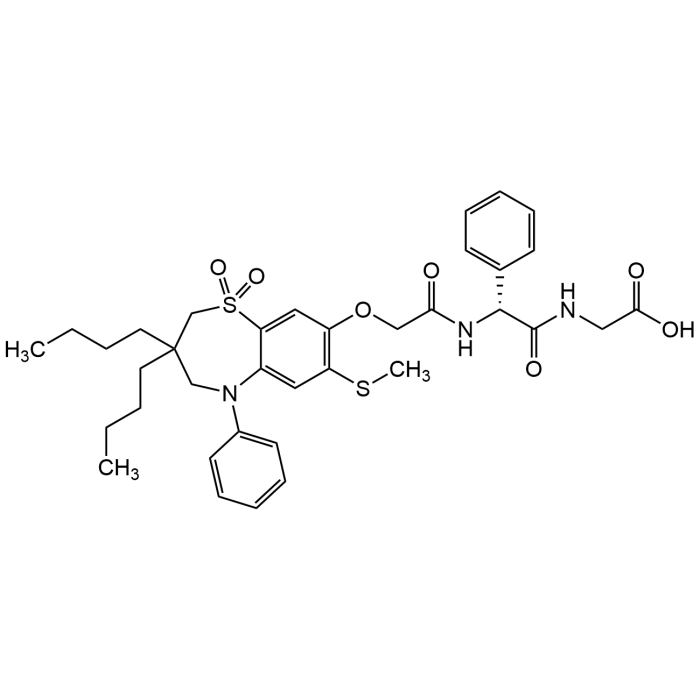
Elobixibat
As low as
902
CHF
CHF 902.00
In stock
Only %1 left
CDX-E0316-M01010 mgCHF 902.00
CDX-E0316-M02525 mgCHF 1’805.00
| Product Details | |
|---|---|
| Synonyms | A3309; AZD 7806; AJG 533; (2R)-N-[2-[[3,3-Dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,5-benzothiazepin-8-yl]oxy]acetyl]-2-phenylglycylglycine |
| Product Type | Chemical |
| Properties | |
| Formula | C36H45N3O7S2 |
| MW | 695.89 |
| CAS | 439087-18-0 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in methanol (5mg/ml). Slightly soluble in DMSO (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | XFLQIRAKKLNXRQ-UUWRZZSWSA-N |
| Smiles | O=S1(=O)C=2C(N(CC(CCCC)(CCCC)C1)C3=CC=CC=C3)=CC(SC)=C(OCC(N[C@@H](C(NCC(O)=O)=O)C4=CC=CC=C4)=O)C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Elobixibat is an inhibitor of the sodium/bile acid and sulfated solute cotransporter (ASBT), also known as the ileal bile acid transporter (IBAT) which is selective for ASBT over the hepatic sodium/bile acid cotransporter. Oral administration of elobixibat reduces methionine- and choline-deficient diet-induced increases in serum bile acid levels and hepatic inflammation, fibrosis, and cytokine gene expression in a mouse model of non-alcoholic steatohepatitis (NASH). Elobixibat lowers LDL cholesterol, increases serum GLP-1, promotes colon motility, and has the potential to treat metabolic syndrome. Elobixibat can be used to study constipation, dyslipidemia, non-alcoholic hepatitis and liver tumors.
Product References
(1) P.-G. Gillberg, et al.; Gastroenterol. 138, S224 (2010) | (2) A. Acosta & M. Camilleri; Therap. Adv. Gastroenterol. 7, 167 (2014) (Review) | (3) M. Rudling, et al.; BMC Cardiovasc. Disord. 15, 75 (2015) | (4) S. Taniguchi, et al.; Neurogastroenterol. Motil. 30, e13448 (2018) | (5) R. Yamauchi, et al.; Hepatol. Int. 15, 392 (2021) | (6) Y. Sugiyama, et al.; Hepatol. Int. 17, 1378 (2023)





