Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Elobixibat hydrate
As low as
77
CHF
CHF 77.00
In stock
Only %1 left
CDX-E0318-M05050 mgCHF 77.00
CDX-E0318-M250250 mgCHF 251.00
| Product Details | |
|---|---|
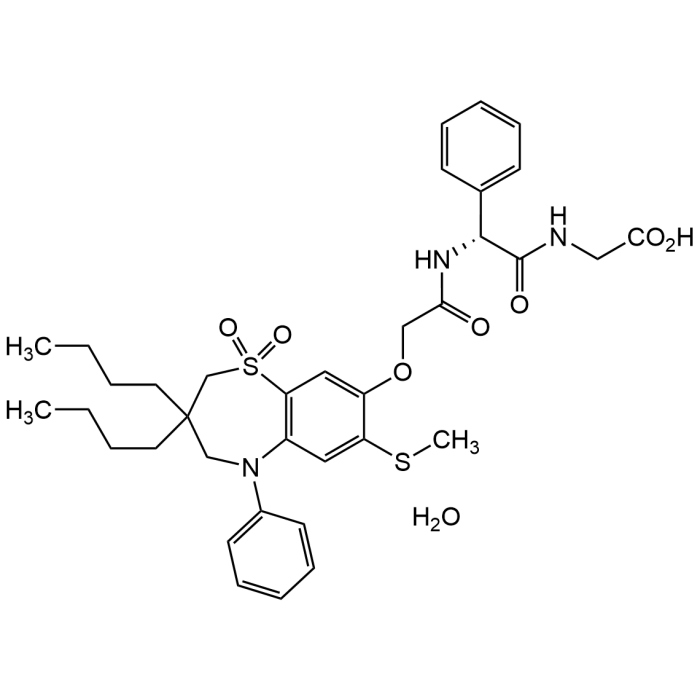
| Synonyms | A3309; Goofice; Glycine, (2R)-N-[2-[[3,3-dibutyl-2,3,4,5-tetrahydro-7-(methylthio)-1,1-dioxido-5-phenyl-1,5-benzothiazepin-8-yl]oxy]acetyl]-2-phenylglycyl-, hydrate (1:1) |
| Product Type | Chemical |
| Properties | |
| Formula | C36H45N3O7S2 . H2O |
| MW | 713.92 |
| CAS | 1633824-78-8 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (30mg/ml) or DMF. Slightly soluble in ethanol. Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VARDBGNECHECBX-MDYNBEAQSA-N |
| Smiles | CCCCC(CN1C2=CC=CC=C2)(CCCC)CS(C3=C1C=C(SC)C(OCC(N[C@@H](C(NCC(O)=O)=O)C4=CC=CC=C4)=O)=C3)(=O)=O.O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Elobixibat is a selective inhibitor of the ileal bile acid transporter (IBAT, also known as ASBT/SLC10A2), used primarily in research on cholesterol metabolism, bile acid regulation, and gastrointestinal motility disorders. By blocking bile acid reabsorption in the terminal ileum, elobixibat increases bile acid flow into the colon, which stimulates fluid secretion and accelerates colonic transit. Elobixibat lowers LDL cholesterol, increases serum GLP-1, promotes colonic motility and has the potential to treat metabolic syndrome. It has been used in studies on chronic idiopathic constipation (CIC), nonalcoholic fatty liver disease (NAFLD) and hyperlipidemia. Elobixibat is a valuable tool for studying enterohepatic circulation, FXR signaling and gut-liver axis interactions.
Product References
(1) A. Acosta & M. Camilleri; Therap. Adv. Gastroenterol. 7, 167 (2014) | (2) P. Mosinska, et al.; World J. Gastroenterol. 21, 7436 (2015) | (3) M. Rudling, et al.; BMC Cardiovasc. Disord. 15, 75 (2015) | (4) Y. Kumagai, et al.; Br. J. Clin. Pharmacol. 84, 2393 (2018) | (5) P.B. Miner; Expert Opin. Pharmacother. 19, 1381 (2018) | (6) R. Yamauchi, et al.; Hepatol. Int. 15, 392 (2021) | (7) S. Yoshinobu, et al.; Clin. Ther. 44, 1418 (2022) | (8) Y. Sugiyama, et al.; Hepatol. Int. 17, 1378 (2023)





