Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
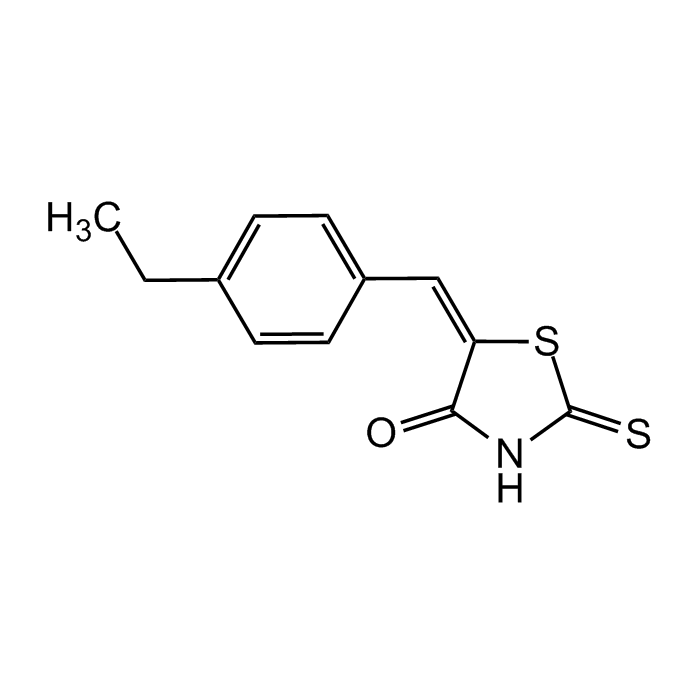
5-[(4-Ethylphenyl)methylene]-2-thioxo-4-thiazolidinone
| Product Details | |
|---|---|
| Synonyms | 10058-F4 |
| Product Type | Chemical |
| Properties | |
| Formula |
C12H11NOS2 |
| MW | 249.35 |
| CAS | 403811-55-2 |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Yellow to dark yellow powder. |
| Solubility | Soluble in DMSO (25mg/ml), ethanol (5mg/ml) or water (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | SVXDHPADAXBMFB-JXMROGBWSA-N |
| Smiles | O=C(NC(S/1)=S)C1=C/C2=CC=C(CC)C=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
c-Myc is a proto-oncogene that plays an important role in cell proliferation, differentiation, and apoptosis. 5-[(4-Ethylphenyl)methylene]-2-thioxo-4-thiazolidinone [10058-F4] is a cell-permeable thiazolidinone compound that specifically inhibits the c-Myc-Max interaction/dimerization at 64 µM, preventing c-Myc-dependent gene expression and cell proliferation. 10058-F4 inhibits tumor cell growth and proliferation in a c-Myc-dependent manner both in vitro and in vivo. 10058-F4 induces cell-cycle arrest (G0/G1 phase) and apoptosis. 10058-F4 has been found to also upregulate cyclin-dependent kinase (CDK) inhibitors, p21 and p27. In addition to c-Myc, 10058-F4 inhibits the nuclear Myc protein, N-Myc, at 50 µM, inducing arrest, apoptosis, and differentiation in neuroblastoma cells overexpressing the gene for N-Myc. This compound can be used to delineate novel actions of Myc proteins, especially those related to lipid and glucose metabolism. 10058-F4 downregulates human telomerase reverse transcriptase and enhances chemosensitivity in several human cancer cell lines.
(1) X. Yin, et al.; Oncogene 22, 6151 (2003) | (2) M.J. Huang, et al.; Exp. Hematol. 34, 1480 (2006) | (3) C.P. Lin, et al.; Anticancer Drugs 18, 161 (2007) | (4) J. Guo, et al.; Cancer Chemother. Pharmacol. 63, 615 (2009) | (5) P. Zhang, et al.; Mol. Endocrinol. 24, 1274 (2010) | (6) H. Zirath, et al.; PNAS 110, 10258 (2013) | (7) M. Zhang, et al.; Biomed. Pharmacother. 73, 123 (2015) | (8) M. Lv, et al.; Mol. Med. Rep. 18, 421 (2018) | (9) N. Sheikh-Zeineddini, et al.; J. Cell Biochem. 120, 14004 (2019) | (10) N. Sheikh-Zeineddini, et al.; Eur. J. Pharmacol. 870, 172821 (2020)





