Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
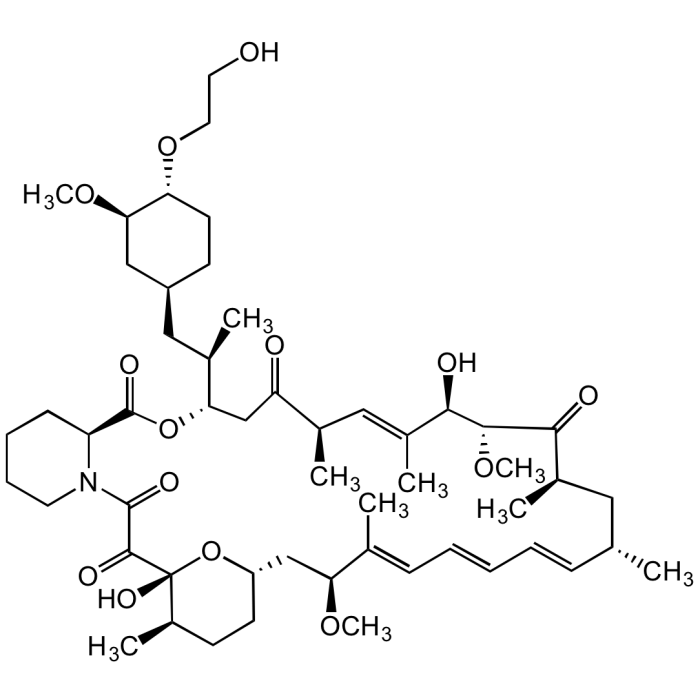
Everolimus Solution
| Product Details | |
|---|---|
| Synonyms | 42-O-(2-Hydroxyethyl)rapamycin; NVP-RAD001; RAD001; SDZRAD; Zortress; Afinitor; Certican; Votubia |
| Product Type | Chemical |
| Properties | |
| Formula |
C53H83NO14 |
| MW | 958.22 |
| CAS | 159351-69-6 |
| RTECS | VE6255000 |
| Purity Chemicals | ≥99% (HPLC) |
| Appearance | Liquid. 1mg/ml in acetonitrile. |
| Identity | Determined by IR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HKVAMNSJSFKALM-QATPHWSPSA-N |
| Smiles | OCCO[C@H]1[C@H](OC)C[C@H](C[C@@H](C)[C@H](CC([C@H](C)/C=C(C)/[C@@H](O)[C@H]2OC)=O)OC([C@@H]3CCCCN3C(C([C@@]4(O)[C@H](C)CC[C@@H](C[C@H](OC)/C(C)=C/C=C/C=C/[C@@H](C)C[C@@H](C)C2=O)O4)=O)=O)=O)CC1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | -20°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Macrolide antibiotic, inhibiting bacterial protein synthesis. Potent immunosuppressant. Binds with high affinity to the FK506 binding protein-12 (FKBP-12) to generate an immunosuppressive complex that inhibits the activation of the mammalian target of rapamycin (mTOR). More selective for the mTORC1 protein complex, with little impact on the mTORC2 complex, compared to Rapamycin. Anticancer agent. Inhibition of mTOR reduces the activity of effectors downstream, which leads to a blockage in the progression of cells from G1 into S phase, and subsequently inducing cell growth arrest, apoptosis and autophagy, resulting in reduction of cell proliferation, angiogenesis and glucose uptake. Inhibits tumor proliferation in vitro and in vivo. This compound can be used as a reference material.
(1) H.J. Schuurman, et al.; Transplantation 64, 32 (1997) | (2) W. Schuler, et al.; Transplantation 64, 36 (1997) | (3) P. Neuhaus, et al.; Liver Transpl. 7, 473 (2001) (Review) | (4) B. Nashan; Expert Opin. Investig. Drugs. 11, 1845 (2002) (Review) | (5) I. Beuvink, et al.; Cell 120, 747 (2005) | (6) J.K. Patel & J.A. Kobashigawa; Expert Opin. Pharmacother. 7, 1347 (2006) (Review) | (7) P. Smolewski; Anticancer Drugs 17, 487 (2006) (Review) | (8) K. Zitzmann, et al.; Neuroendocrinology 85, 54 (2007) | (9) Z. Zeng, et al.; Blood 109, 3509 (2007) | (10) R. Bianco, et al.; Br. J. Cancer 98, 923 (2008) | (11) A.I. Sanchez-Fructuoso; Expert Opin. Drug Metab. Toxicol. 4, 807 (2008) (Review) | (12) H.A. Lane, et al.; Clin. Cancer Res. 15, 1612 (2009) | (13) D. Lebwohl, et al.; Ann. N. Y. Acad. Sci. 1291, 14 (2013) (Review) | (14) U. Saran, et al.; Clin. Sci. 129, 895 (2015) (Review) | (15) Morviducci, et al.; Diabetes Res. Clin. Pract. (Epub ahead of print) (2018) (Review)





