Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
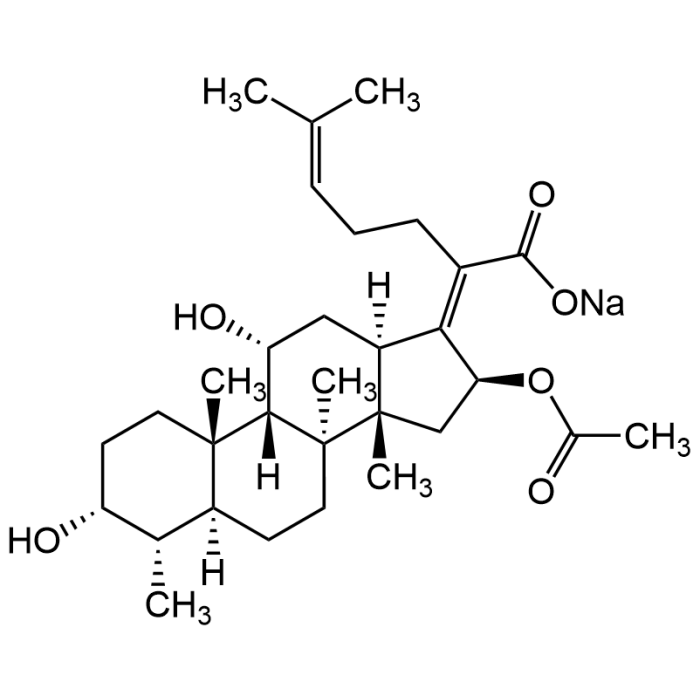
Fusidic acid sodium salt
As low as
238
CHF
CHF 238.00
In stock
Only %1 left
CDX-F0118-G0011 gCHF 238.00
| Product Details | |
|---|---|
| Synonyms | Fucidin; SQ 16360; Fusidate sodium |
| Product Type | Chemical |
| Properties | |
| Formula | C31H47O6Na |
| MW | 538.69 |
| CAS | 751-94-0 |
| RTECS | LV5775000 |
| Source/Host Chemicals | Microbial |
| Purity Chemicals | ≥98% (TLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water or DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HJHVQCXHVMGZNC-JCJNLNMISA-M |
| Smiles | O=C(O[Na])/C(CC/C=C(C)/C)=C1[C@@H](OC(C)=O)C[C@]2(C)[C@@]3(C)CC[C@@]4([H])[C@H](C)[C@H](O)CC[C@]4(C)[C@]3([H])[C@H](O)C[C@]2\1[H] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
The tetracyclic triterpenoid is a fusidane antibiotic originally isolated from F. coccineum. It is active against the Gram-positive bacteria S. aureus, S. pyogenes, C. diphtheriae, B. subtilis, and C. tetani but not the Gram-negative bacteria E. coli, S. typhimurium, and P. vulgaris or the fungi C. albicans and A. fumigatus. This antibacterial agent inhibits bacterial protein synthesis by preventing the release of translation elongation factor G (EF-G) from ribosomes. Fusidic acid has immunomodulatory effects and inhibits the inhibitory and activating effects of interleukins IL-1 and IL-6 on glucose-induced insulin production and exhibits antidiabetic effects in a rat model. Fusidic acid improves the symptoms of colitis in rats and inhibits the growth of Toxoplasma gondii and Listeria monocytogenes EGD in vitro, but not in mice. The sodium salt version is found to be more water-soluble.
Product References
(1) W.O. Godtfredsen, et al.; Nature 193, 987 (1962) | (2) N. Tanaka, et al.; Biochem. Biophys. Res. Commun. 30, 278 (1968) | (3) L. Verbist; J. Antimicrob. Chemother. 25, 1 (1990) | (4) K. Bendtzen, et al.; J. Endocrinol. 132, 345 (1992) | (5) K. Buschard, et al.; Autoimmunity 14, 101 (1992) | (6) J Turnidge, et al.; Int. J. Antimicrob. Agents 12, S23 (1999) | (7) P. Collignon & L. Turnidge; Int. J. Antimicrob. Agents 12, S45 (1999) | (8) K. Christiansen; Int. J. Antimicrob. Agents 12, S73 (1999) | (9) A. Savelsbergh, et al.; RNA 15, 772 (2009)





