Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
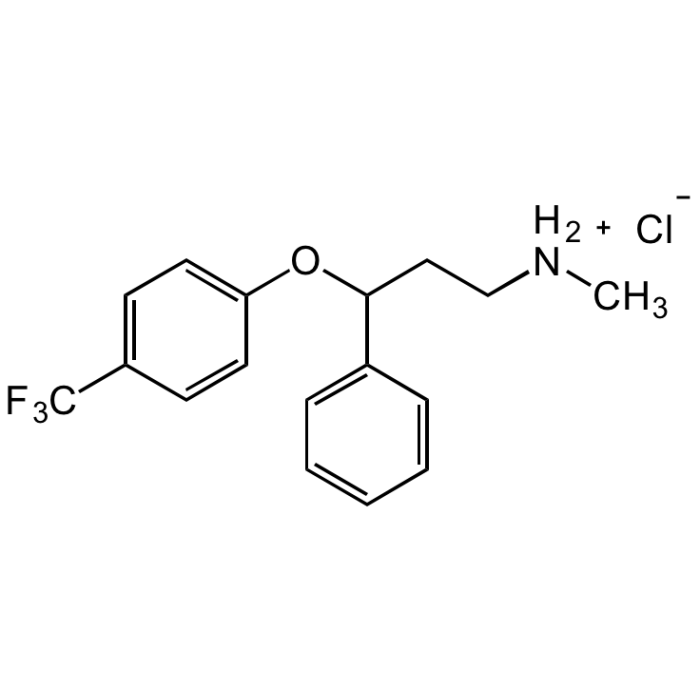
Fluoxetine hydrochloride
| Product Details | |
|---|---|
| Synonyms | (±)-N-Methyl-γ-[4-(trifluoromethyl)phenoxy]benzenepropanamine hydrochloride; LY-110,140 hydrochloride; Prozac |
| Product Type | Chemical |
| Properties | |
| Formula |
C17H18F3NO . HCl |
| MW | 345.79 |
| CAS | 56296-78-7 |
| RTECS | UI4050000 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in water (2mg/ml), DMSO (10mg/ml), DMF (10mg/ml) or ethanol (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | GIYXAJPCNFJEHY-UHFFFAOYSA-N |
| Smiles | C[NH2+]CCC(C1=CC=CC=C1)OC2=CC=C(C(F)(F)F)C=C2.[Cl-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Fluoxetine is a cell-permeable selective serotonin reuptake inhibitor (SSRI), with preference for the serotonin transporter (Kd=0.81nM) over the norepinephrine transporter (Kd=240nM) and the dopamine transporter (Kd=3600nM). This drug works at presynaptic terminals where it prevents the reuptake of serotonin, resulting in the accumulation of serotonin in extracellular fluid at synapses. It functions as an antidepressant. Fluoxetine binds also to the human 5-HT transporter and is between 150- and 900- fold selective over 5-HT1A, 5-HT2A, H1, α1, α2-adrenergic, and muscarinic receptors. It has been shown to induce differentiation of neuronal precursors, enhancing neuronal characteristics. In addition, it was reported to modulate the proliferation of T-cells by increasing the Ca2+ influx and thereby influencing the activities of protein kinase A (PKA) and protein kinase C (PKC) and to regulate the phosphorylation of DARPP-32 and AMPA receptors.
(1) P. Benfield, et al.; Drugs 32, 481 (1986) | (2) D.T. Wong, et al.; Life Sci. 57, 411 (1995) | (3) M. Tatsumi, et al.; Eur. J. Pharmacol 340, 249 (1997) | (4) S.G. Beck, et al.; J. Pharmacol. Exp. Ther. 281, 115 (1997) | (5) M.J. Owens, et al.; J. Pharmacol. Exp. Ther. 283, 1305 (1997) | (6) V.A. Edgar, et al.; Eur. J. Pharmacol. 372, 65 (1999) | (7) P. Bartholoma, et al.; Biochem. Pharmacol. 63, 1507 (2002) | (8) J.T. Bian, et al.; Eur. J. Pharmacol. 453, 159 (2002) | (9) A. Zhang, et al.; J. Med. Chem. 45, 1930 (2002) | (10) P. Svenningsson, et al.; PNAS 99, 3182 (2002) | (11) L. Perez-Caballero, et al.; Expert Opin. Drug Discov. 9, 567 (2014)





