Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Forskolin
| Product Details | |
|---|---|
| Synonyms | Boforsin; Colforsin; Coleonol; NSC 357088; NSC 375489; HL 362; L 75-1362B |
| Product Type | Chemical |
| Properties | |
| Formula |
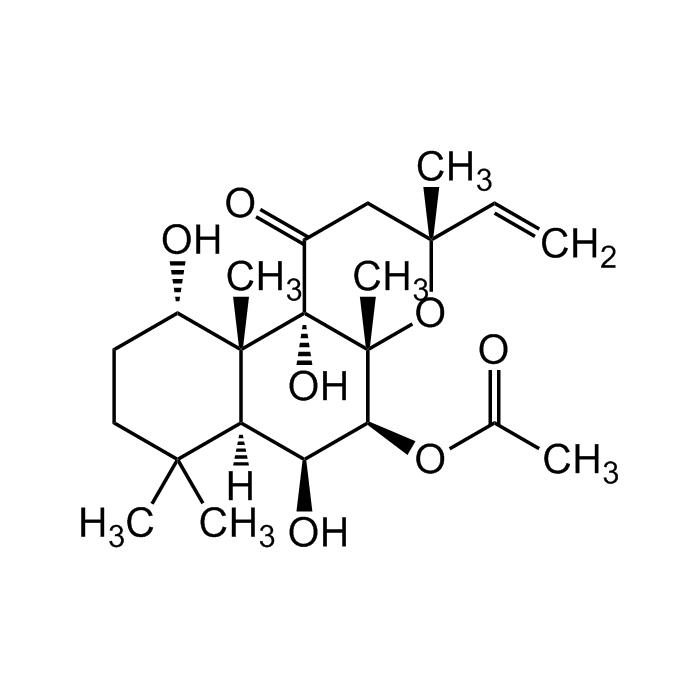
C22H34O7 |
| MW | 410.5 |
| CAS | 66575-29-9 |
| RTECS | QL6150000 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO, DMF or ethanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | OHCQJHSOBUTRHG-KGGHGJDLSA-N |
| Smiles | C=C[C@](C1)(C)O[C@]2(C)[C@@H](OC(C)=O)[C@@H](O)[C@@]3([H])C(C)(C)CC[C@H](O)[C@]3(C)[C@@]2(O)C1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Forskolin is a naturally occurring diterpene. It directly activates adenylyl cyclase through its catalytic subunit and is commonly used to raise levels of cAMP (an important signal carrier necessary for the proper biological response of cells to hormones, neurotransmitters, and other extracellular signals) in a wide variety of intact cells and tissue preparations. Forskolin also interacts with glucose transporters and certain ion channels and has been used for examining adenylyl cyclase expression, regulation, and G protein signaling. Forskolin is a smooth muscle relaxant/vasodilator with inotropic and antihypertensive activity. Forskolin has antioxidant and anti-inflammatory property and reduces hyperglycemia by stimulating insulin release. It has anti-neuroinflammatory and neuroprotective properties that ameliorates Alzheimer’s disease symptoms and has also shown a number of antitumor effects.
(1) H. Metzger & E. Lindner; Arzneimittelforschung 31, 1248 (1981) | (2) K.B. Seamon & J.W. Daly; J. Cyclic Nucleotide Res. 7, 201 (1981) | (3) N.J. de Souza, et al.; Med. Res. Rev. 3, 201 (1983) | (4) H.G. Joost & H.J. Steinfelder; Mol. Pharmacol. 31, 279 (1987) | (5) A. Laurenza, et al.; TIPS 10, 442 (1989) (Review) | (6) M.L. Aylwin & M.M. White; Mol. Pharmacol. 41, 908 (1992) | (7) P.J. Scarpace & M. Matheny; Pflugers Arch. 431, 388 (1996) | (8) P.A. Insel & R.S. Ostrom; Cell Mol. Neurobiol. 23, 305 (2003) | (9) N. Canu, et al.; J. Neurochem. 92, 1228 (2005) | (10) H. Yamanaka, et al.; Mol. Med. Rep. 3, 133 (2010) | (11) R.H. Alasbahi & M.F. Melzig; Pharmazie 67, 5 (2012) (Review) | (12) V.D. Wagh, et al.; J. Postgrad. Med. 58, 199 (2012) (Review) | (13) B.A. Owona, et al.; J. Neuropathol. Exp. Neurol. 75, 618 (2016) | (14) L. Sapio, et al.; J. Cell Physiol. 232, 922 (2017) (Review)





