Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
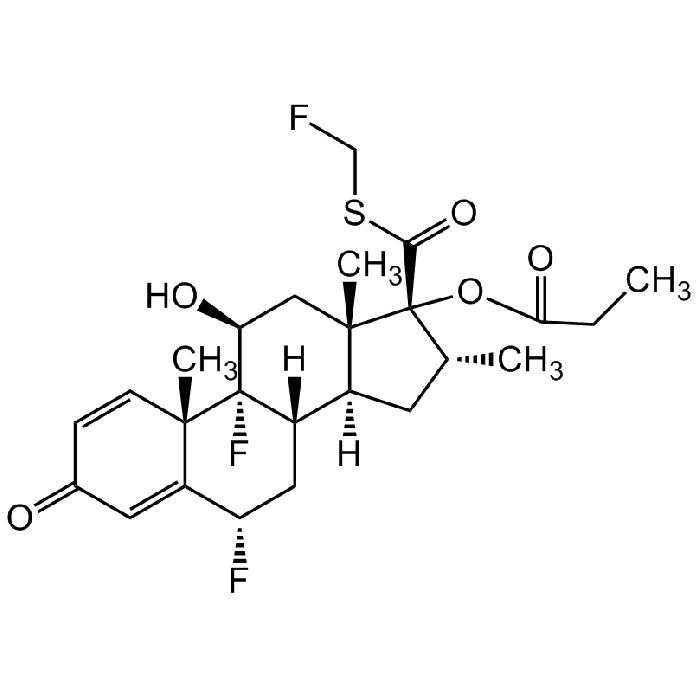
Fluticasone propionate
| Product Details | |
|---|---|
| Synonyms | Fluticasone 17-Propionate; (6α,11β,16α,17α)-6,9-Difluoro-11-hydroxy-16-methyl-3-oxo-17-(1-oxopropoxy)androsta-1,4-diene-17-carbothioic acid S-(fluoromethyl) ester; CCI 18781 |
| Product Type | Chemical |
| Properties | |
| Formula |
C25H31F3O5S |
| MW | 500.57 |
| CAS | 80474-14-2 |
| RTECS | BV7980000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥97% (NMR) |
| Appearance | White powder. |
| Solubility | Soluble in DMSO (20mg/ml) or DMF (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | WMWTYOKRWGGJOA-CENSZEJFSA-N |
| Smiles | O[C@H]1C[C@@]2(C)[C@](C[C@@H](C)[C@]2(OC(CC)=O)C(SCF)=O)([H])[C@]3([H])C[C@H](F)C4=CC(C=C[C@]4(C)[C@]31F)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Fluticasone propionate is a synthetic corticosteroid. It is a high affinity, selective glucocorticoid receptor agonist (Kd = 0.5nM). It is used as an anti-inflammatory agent for asthma. It has low systemic bioavailability and has been investigated for effectiveness as an inhaled or intranasal corticosteroid to reduce airflow obstruction related to asthma, allergic rhinitis and other airway diseases. It potently stimulates glucocorticoid receptor-mediated transactivation of gene expression and enhances human eosinophil apoptosis in vitro. It also potentiates KV1 channels (EC50 = 37nM).
(1) M. Johnson; Allergy 50, 11 (1995) (Review) | (2) M. Johnson; Int. Arch. Allergy Immunol. 107, 439 (1995) (Review) | (3) R. Fuller, et al.; Respir. Med. 89, 3 (1995) (Review) | (4) C.L. Smith & W. Kreutner; Arzneim.-Forsch. 48, 956 (1998) | (5) X. Zhang, et al.; Eur. J. Pharmacol. 406, 325 (2000) | (6) Y. Pan, et al.; ACS Chem Biol. 7, 1641 (2012) | (7) J. Sastre & R. Mosges; J. Investig. Allergol. Clin. Immunol. 22, 1 (2012) | (8) J. Gonzalez-Cervera & A.J. Lucendo; J. Investig. Allergol. Clin. Immunol. 26, 8 (2016)





