Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
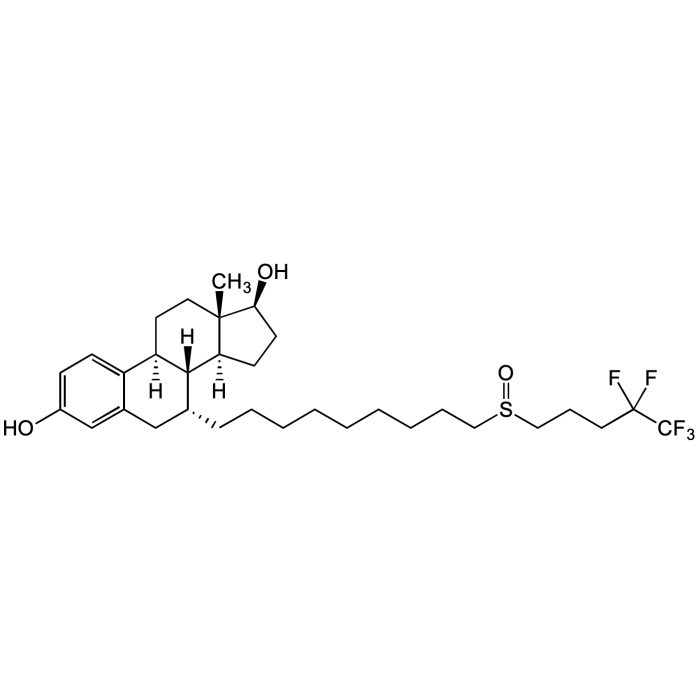
Fulvestrant
| Product Details | |
|---|---|
| Synonyms | ICI 182780; Faslodex; 7α-[9-[(RS)-(4,4,5,5,5-Pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene-3,17beta-diol |
| Product Type | Chemical |
| Properties | |
| Formula | C32H47F5O3S |
| MW | 606.77 |
| CAS | 129453-61-8 |
| RTECS | KG7623000 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White or off white crystalline powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF (20mg/ml) or ethanol (20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VWUXBMIQPBEWFH-WCCTWKNTSA-N |
| Smiles | OC1=CC=C2C(C[C@@H](CCCCCCCCCS(CCCC(F)(F)C(F)(F)F)=O)[C@]3([H])[C@]2([H])CC[C@@]4(C)[C@@]3([H])CC[C@@H]4O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Fulvestrant is a selective estrogen receptor degrader (SERD) that downregulates and degrades estrogen receptor α (ERα). It reduces dimerization and nuclear localization of the estrogen receptor (ER). Fulvestrant lowers the level of ER protein in human breast cancer cells. Fulvestrant inhibits the growth of ER-positive MCF-7 human breast cancer cells but not ER-negative BT-20 cells when used at a concentration of 1μg/ml. It also prevents tumor growth in MCF-7 and Br10 breast cancer mouse xenograft models when used at a single dose of 5 mg per animal. Fulvestrant is neuroprotective in vitro against neurotoxicity induced by glutamate- and amyloid-β (1-42) (Aβ42) in primary rat hippocampal cells. Formulations containing fulvestrant have been used in the treatment of estrogen-sensitive breast cancer.
(1) A.E Wakeling, et al.; Cancer Res. 51, 3867 (1991) | (2) J.F. Robertson; Br. J. Cancer 85, 11 (2001) (Review) | (3) N. Bundred & A. Howell; Expert Rev. Anticancer Ther. 2, 151 (2002) (Review) | (4) C.K. Osborne, et al.; Br. J. Cancer 90, S2 (2004) (Review) | (5) S. Kansra, et al.; Mol. Cell Endocrinol. 239, 27 (2005) | (6) M. Dowsett, et al.; Breast Cancer Res. Treat. 93, S11 (2005) (Review) | (7) L. Zhao, et al.; J. Pharmacol. Exp. Ther. 319, 1124 (2006) | (8) P. Kabos & V.F. Borges; Expert Opin. Pharmacother. 11, 807 (2010) (Review) | (9) S.E. Wardell, et al.; Biochem. Pharmacol. 82, 122 (2011)





