Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
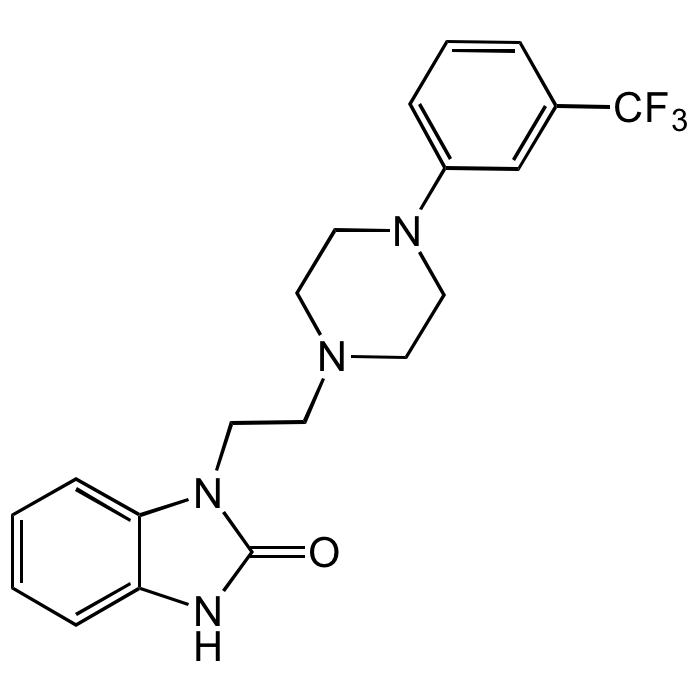
Flibanserin
| Product Details | |
|---|---|
| Synonyms | 1,3-Dihydro-1-[2-[4-[3-(trifluoromethyl)phenyl]-1-piperazinyl]ethyl]-2H-benzimidazol-2-one; BIMT 17; BIMT 17BS |
| Product Type | Chemical |
| Properties | |
| Formula | C20H21F3N4O |
| MW | 390.4 |
| CAS | 167933-07-5 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to beige powder. |
| Solubility | Soluble in DMSO (20mg/ml), DMF (20mg/ml) or ethanol (10mg/ml) |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PPRRDFIXUUSXRA-UHFFFAOYSA-N |
| Smiles | O=C1NC2=CC=CC=C2N1CCN3CCN(C4=CC=CC(C(F)(F)F)=C4)CC3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Flibanserin is a full agonist of the serotonin 5-HT1A receptor and an antagonist of 5-HT2A (Ki=1nm and 49nM, respectively). It also binds to dopamine D4 receptors with Ki values ranging from 4-24nM, but demonstrates no affinity for the other 5-HT subtypes or other neurotransmitter receptors. Multifunctional serotonergic ligands like flibanserin that can enhance downstream release of dopamine and norepinephrine while concomitantly reducing serotonin release in the brain circuits that mediate symptoms of reduced sexual interest and desire, are being studied clinically for therapeutic potential to improve sexual functioning. Flibanserin was investigated as a novel, non-hormonal treatment for pre-menopausal women with Hypoactive Sexual Desire Disorder (HSDD). Flibanserin was also found to reduce L-DOPA-induced dyskinesia in a model of Parkinson‘s Disease.
(1) F. Borsini, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 352, 276 (1995) | (2) F. Borsini, et al.; Naunyn Schmiedebergs Arch. Pharmacol. 352, 283 (1995) | (3) F. Borsini, et al.; Psychopharmacology 134, 378 (1997) | (4) A. Scandroglio, et al.; Pharmacol. Res. 43, 179 (2001) | (5) F. Borsini, et al.; CNS Drug Rev. 8, 117 (2002) (Review) | (6) R.W. Invernizzi, et al.; Br. J. Pharmacol. 139, 1281 (2003) | (7) H. Gelez, et al.; J. Sex. Med. 10, 1231 (2013) | (8) S.M. Stahl; CNS Spectr. 20, 1 (2015) (Review) | (9) I. Dhanuka & J.A. Simon; Expert Opin. Pharmacother. 16, 2523 (2015) (Review)





