Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
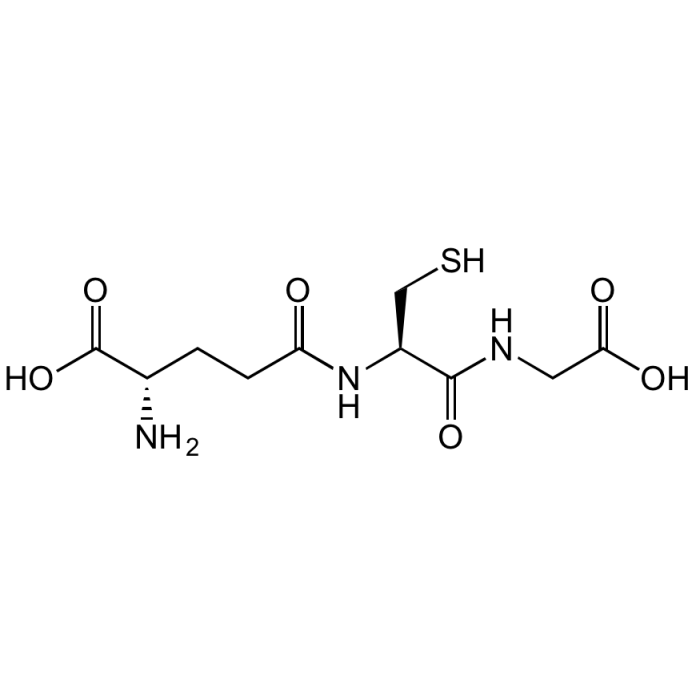
L-Glutathione reduced
| Product Details | |
|---|---|
| Synonyms | GSH; L-γ-glutamyl-L-cysteinyl-glycine |
| Product Type | Chemical |
| Properties | |
| Formula | C10H17N3O6S |
| MW | 307.32 |
| CAS | 70-18-8 |
| Purity Chemicals | ≥98% (Titration) |
| Appearance | White crystalline powder. |
| Solubility | Soluble in water (20 mg/ml) or PBS (10mg/ml). Sparingly soluble in DMSO or ethanol. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | RWSXRVCMGQZWBV-WDSKDSINSA-N |
| Smiles | OC([C@H](CCC(N[C@@H](CS)C(NCC(O)=O)=O)=O)N)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Major tripeptide widely distributed in both plants and animals. Endogenous antioxidant that plays a major role in reducing reactive oxygen species formed during cellular metabolism and the respiratory burst. Regulates activity of the redox sensitive transcription factor NF-κB. Cytoprotective. Serves as a nucleophilic co-substrate to glutathione transferases in the detoxification of xenobiotics and is an essential electron donor to glutathione peroxidases in the reduction of hydroperoxides. Involved in amino acid transport and maintenance of protein sulfhydryl reduction status. Posseses several metabolic, regulatory and protective functions. Forms disulfide bonds with cysteine residues in proteins.
(1) A. Pompella, et al.; Biochem. Pharmacol. 66, 1499 (2003) | (2) A. Pastore, et al.; Clin. Chim. Acta. 333, 19 (2003) | (3) A. Chatterjee; Nutrients 5, 525 (2013) | (4) E.V. Kalinina, et al.; Biochemistry 79, 1562 (2014) | (5) K. Aquilano, et al.; Front. Pharmacol. 5, 196 (2014) | (6) P. Diaz-Vivancos, et al.; Free Radic. Biol. Med. 89, 1154 (2015) | (7) T. Homma & J. Fujii; Curr. Drug Metab. 16, 560 (2015)





