Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
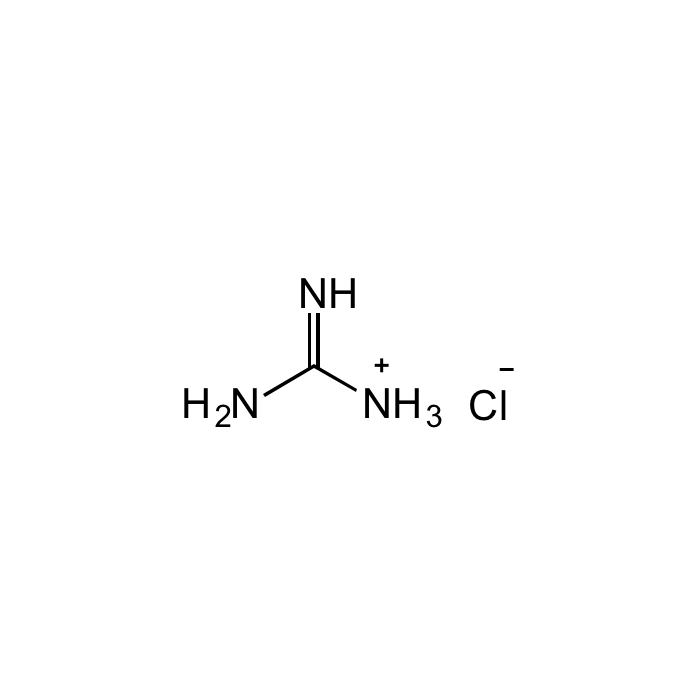
Guanidine hydrochloride
| Product Details | |
|---|---|
| Synonyms | Aminoformamidine hydrochloride; Aminomethanamidine hydrochloride; Guanidinium chloride; Carbamimidoylazanium chloride |
| Product Type | Chemical |
| Properties | |
| Formula | CH5N3 . HCl |
| MW | 59.07 . 36.46 |
| CAS | 50-01-1 |
| RTECS | MF4300000 |
| Purity Chemicals | ≥99% (Titration) |
| Appearance | White powder. |
| Solubility | Soluble in water (6M) or ethanol (1M). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PJJJBBJSCAKJQF-UHFFFAOYSA-N |
| Smiles | NC([NH3+])=N.[Cl-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Guanidine hydrochloride is a powerful, chaotropic agent which is widely used for purification of proteins and nucleic acids. It is useful for denaturation and refolding of proteins as well as in the recovery of periplasmic proteins. At higher concentrations, this strong denaturant has been reported to solubilize denatured insoluble proteins such as inclusion bodies and to increase the solubility of hydrophobic molecules. When utilized at lower concentrations, it is useful in prompting the refolding of denatured proteins. Guanidine hydrochloride is also been reported to allow return of enzymatic activity in protocols using it as a first step. Guanidine hydrochloride is used in RNA isolation to dissociate nucleoproteins and inhibit RNase.
(1) C. Holm, et al.; Gene 42, 169 (1986) | (2) C.N. Pace, et al.; Biochemistry 29, 2564 (1990) | (3) F.A. Marston, et al.; Meth. Enzymol. 182, 264 (1990) | (4) R. Rudolph & H. Lilie; FASEB J. 10, 49 (1996) | (5) A. Mukhopadhyay; Adv. Biochem. Eng. Biotechnol. 56, 61 (1997) | (6) S. Emadi & M. Behzadi; BBRC 450, 1339 (2014)





