Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Gentamicin sulfate
As low as
271
CHF
CHF 271.00
In stock
Only %1 left
CDX-G0050-G01010 gCHF 271.00
CDX-G0050-G05050 gCHF 812.00
| Product Details | |
|---|---|
| Synonyms | Apogen; Garamycin; Gentiomycin C; Refobacin; NSC-82261 |
| Product Type | Chemical |
| Properties | |
| Formula | n/a |
| MW | n/a |
| CAS | 1405-41-0 |
| Source/Host Chemicals | Microbial |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water (50 mg/ml). |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | RDEIXVOBVLKYNT-HDZPSJEVSA-N |
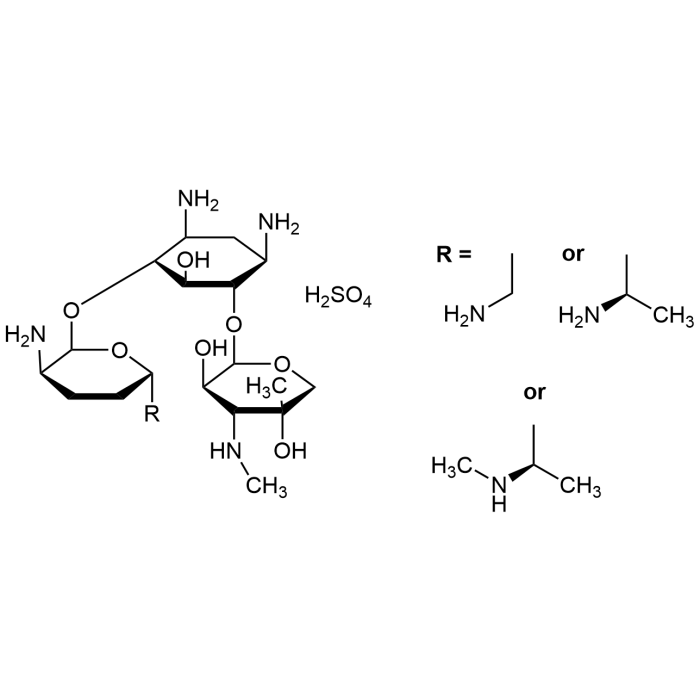
| Smiles | O=S(O)(O)=O.O[C@]1(C)[C@@H]([C@@H](O)[C@@H](O[C@@H]2[C@@H](O)[C@H](O[C@H]3O[C@@](CC[C@H]3N)([C@@H](C)NC)[H])[C@@H](N)C[C@H]2N)OC1)NC.O[C@]4(C)[C@@H]([C@@H](O)[C@@H](O[C@@H]5[C@@H](O)[C@H](O[C@H]6O[C@@](CC[C@H]6N)([C@@H](C)N)[H])[C@@H](N)C[C@H]5N)OC4)NC.O[ |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep under inert gas. Protect from light and oxygen. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Gentamicin sulfate is a broad-spectrum aminoglycoside antibiotic used to treat a wide range of bacterial infections. It is antibacterial against Gram-negative aerobic bacteria, Gram-positive bacteria and mycoplasmas. Gentamicin sulfate is a protein synthesis inhibitor. It causes codon misreading by binding to the 30S ribosomal subunit, blocking the translocation of peptidyl-tRNA from the acceptor site to the donor site. Gentamicin sulfate is used as a selection agent (gentamicin-resistance gene) in molecular biology applications. It is a broad-spectrum cell culture antibiotic that is nontoxic to viruses and mammalian cells at antibacterial and antimycoplasmal concentrations. Due to its extended stability and slow development of bacterial resistance, it is a useful antibiotic in long-term virus und tissue culture studies.
Product References
(1) M. Finland; Med. Times 97, 161 (1969) (Review) | (2) R.E. Hancock; J. Antimicrob. Chemother. 8, 249 (1981) (Review) | (3) E. Hancock; J. Antimicrob. Chemother. 8, 429 (1981) (Review) | (4) N.L. Martin & T.J. Beveridge; Antimicrob. Agents Chemother. 29, 1079 (1986) | (5) J.P. Montenez, et al.; Toxicol. Lett. 73, 201 (1994) | (6) F.C. Luft; J. Mol. Med. 80, 543 (2002) (Review) | (7) Y. Quiros, et al.; Toxicol. Sci. 119, 245 (2011) (Review)





