Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
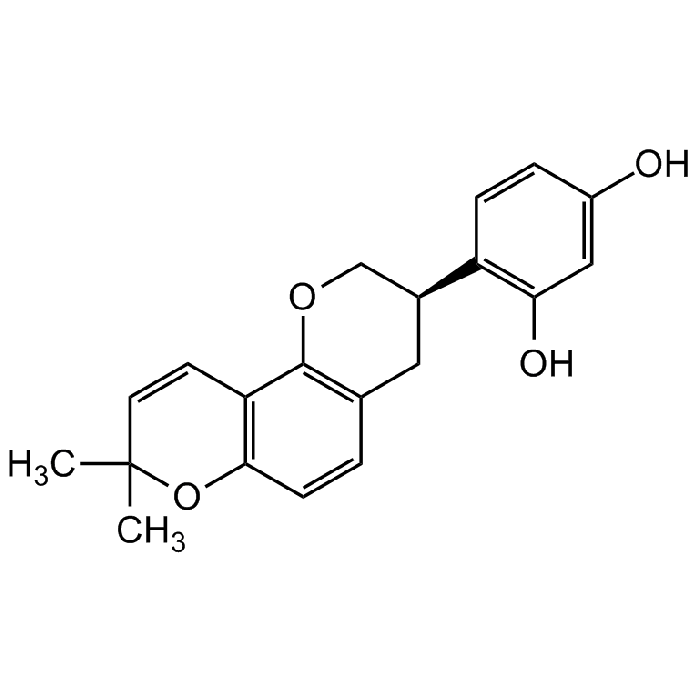
Glabridin
| Product Details | |
|---|---|
| Synonyms | (R)-4-(3,4-Dihydro-8,8-dimethyl-2H,8H-benzo[1,2-b:3,4-b']dipyran-3-yl)-1,3-benzenediol |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H20O4 |
| MW | 324.37 |
| CAS | 59870-68-7 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to light brown powder. |
| Solubility | Soluble in DMSO, ethanol or DMF (all 20mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | LBQIJVLKGVZRIW-ZDUSSCGKSA-N |
| Smiles | CC1(C)C=CC2=C(C=CC3=C2OC[C@@H](C4=C(O)C=C(O)C=C4)C3)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Glabridin is a prenylated isoflavonoid found in licorice (Glycyrrhiza glabra L.) root extract that is reported to possess antiviral, antimicrobial, anti-inflammatory, antidiabetic, antiatherogenic, antioxidant, antitumor and estrogen-like properties. These diverse biological activities are largely related to the capacity of glabridin to down-regulate reactive oxygen species, bind to antioxidant effectors and act as a selective estrogen receptor modulator. On a molecular level it is, next to other targets, activating PPARγ, inhibiting JNK1/2 and human carboxylesterase 2 (CES2A). Based on its antioxidant capacity it is also studied as an anti-aging cosmetic agent. Recently, it has been shown in docking experiments to have high binding affinity to the main protease of SARS-CoV-2.
(1) C. Simmler, et al.; Fitoterapia 90, 160 (2013) | (2) L. Wang, et al.; Acta. Pharmac. Sin. B 5, 310 (2015) | (3) K.J. Lee, et al.; Evid.-Based Compl. Altern. Med. 2015, ID 165457 (2015) | (4) J.F. Rebhun, et al.; Fitoterapia 106, 55 (2015) | (5) C.-T. Chen, et al.; Environ. Toxicol. 33, 679 (2018) | (6) Y.-Q. Song, et al.; Int. J. Biol. Macromol. 137, 261 (2019) | (7) P. Ciganovic, et al.; Antioxidants 8, 445 (2019) | (8) Z.-F. Wang, et al.; Curr. Med. Chem. 27, 1997 (2020) (Review) | (9) R. Islam, et al.; J. Biomol. Struct. Dyn. (Epub ahead of Print) (2020) | (10) B. Goel, et al.; Nat. Prod. Res. (Epub ahead of Print) (2020)





