Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
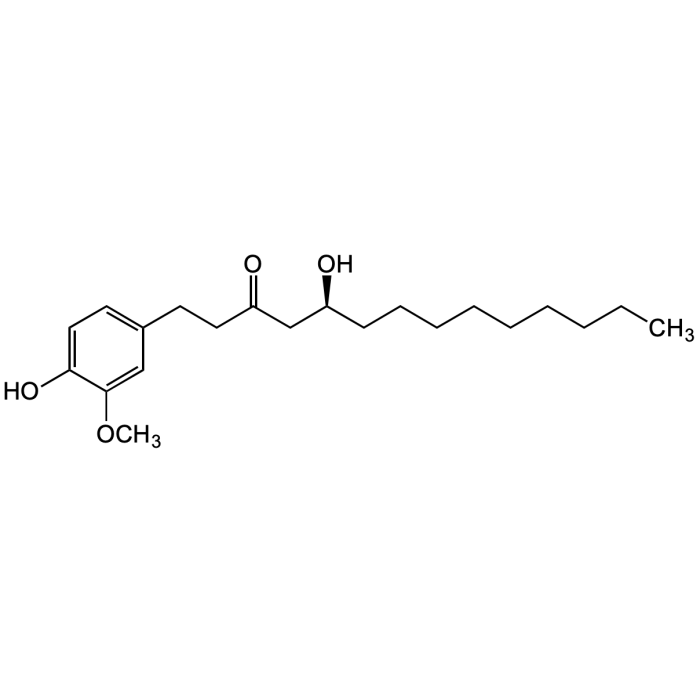
[10]-Gingerol
| Product Details | |
|---|---|
| Synonyms | (S)-5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-tetradecanone; 10-Gingerol; (S)-[10]-Gingerol |
| Product Type | Chemical |
| Properties | |
| Formula | C21H34O4 |
| MW | 350.49 |
| CAS | 23513-15-7 |
| RTECS | XB8720000 |
| Source/Host Chemicals | Isolated from plant source. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Light-yellow powder. |
| Solubility | Soluble in DMSO (25mg/ml), DMF (30mg/ml), ethanol (15mg/ml) or methanol (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AIULWNKTYPZYAN-SFHVURJKSA-N |
| Smiles | OC1=CC=C(CCC(C[C@@H](O)CCCCCCCCC)=O)C=C1OC |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
10-Gingerol is originally found in species of Zingiber. It exhibits antiemetic, anticancer, antioxidative, anti-inflammatory and antibiotic activities. 10-Gingerol increases radical scavenging of superoxide and hydroxyl radicals, inhibits oxidative burst activity and decreases expression of NO and PGE2 in vitro. It exhibits antioxidant behaviour towards lipids and is effective in suppressing obesity and adipose tissue inflammation. 10-Gingerol also inhibits 5-HT3 receptors. Additionally, 10-gingerol inhibits proliferation in different cancer cells. Shwon to suppresses IL-2-induced proliferation of T lymphocytes. This compound also displays antibacterial efficacy against gram negative bacteria such as Porphyromonas and Prevotella, antifungal and antiparasitic activity.
(1) S. Chrubasik, et al.; Phytomedicine 12, 684 (2005) | (2) H. Abdel-Aziz, et al.; Eur. J. Pharmacol. 530, 136 (2006) | (3) M. Park, et al.; Phytother. Res. 22, 1446 (2008) | (4) C.Y. Chen, et al.; Molecules 14, 959 (2009) | (5) S. Dugasani, et al.; J. Ethnopharmacol. 127, 515 (2010) | (6) S.C. Ho, et al.; Food Chem. 141, 3183 (2013) | (7) M.A. Bakht, et al.; Asian Pac. J. Trop. Med. 4, 329 (2014) | (8) M. Bernard, et al.; Phytother. Res. 29, 1707 (2015) | (9) M.M. Bernard, et al.; Exp. Mol. Pathol. 102, 370 (2017) | (10) F. Zhang, et al.; Food Funct. 8, 2635 (2017) (Review) | (11) W. Si, et al.; Food Chem. 239, 1117 (2018) | (12) A.M. Fuzer, et al.; Anticancer Agents Med. Chem. 19, 645 (2019) | (13) Y.W. Fu, et al.; Vet. Parasitol. 265, 74 (2019)





