Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
Glimepiride
| Product Details | |
|---|---|
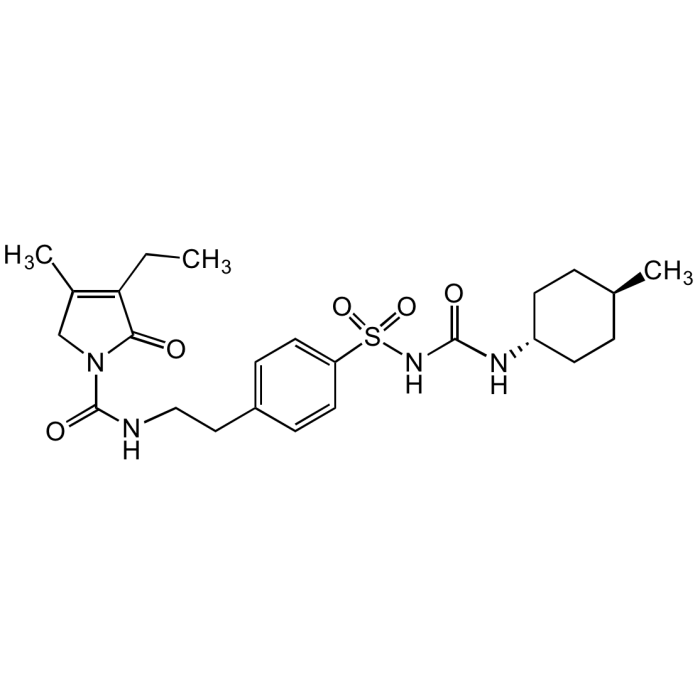
| Synonyms | trans-3-Ethyl-2,5-dihydro-4-methyl-N-[2-[4-[[[[trans-4-methylcyclohexyl)amino]carbonyl]amino]sulfonyl]phenyl]ethyl]-2-oxo-1H-pyrrole-1-carboxyamide; Amaryl; HOE 490 |
| Product Type | Chemical |
| Properties | |
| Formula |
C24H34N4O5S |
| MW | 490.62 |
| CAS | 93479-97-1 |
| RTECS | UX9363950 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White solid. |
| Solubility | Soluble in DMSO (10mg/ml) or DMF (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | WIGIZIANZCJQQY-RUCARUNLSA-N |
| Smiles | O=C(N1C(C(CC)=C(C)C1)=O)NCCC2=CC=C(S(NC(N[C@H]3CC[C@H](C)CC3)=O)(=O)=O)C=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Glimepiride is a long-acting sulfonylurea antidiabetic agent inhibiting ATP-sensitive potassium (KATP) channels in pancreatic β-cells, which leads to the release of insulin. In addition, Glimepiride increases the activity of intracellular insulin receptors. Studies conducted on adipocytes and skeletal muscle suggest that Glimepiride induces the PI3 kinase (PI3K) and Akt pathway, along with insulin receptor substrate-1/2 and endothelial nitric oxide synthase and stimulates glucose transporter 1 and 4 (GLUT1/4) expression. Glimepiride also increases osteoblast proliferation and differentiation, which is thought to be related to its ability to activate the PI3K and Akt pathway and exhibits neuroprotective benefit, decreasing expression and activity of BACE1 and amyloid-β (Aβ) in neurons in a PPARγ-dependent manner.
(1) W. Kramer, et al.; Horm. Metab. Res. 28, 464 (1996) | (2) G. Muller & K. Geisen; Horm. Metab. Res. 28, 469 (1996) | (3) D.K. Song & F.M. Ashcroft; Br. J. Pharmacol. 133, 193 (2001) | (4) M.L. Hribal, et al.; Mol. Pharmacol. 59, 322 (2001) | (5) C.L. Lawrence, et al.; Br. J. Pharmacol. 136, 746 (2002) | (6) K. Inukai, et al.; BBRC 328, 484 (2005) | (7) P. Ma, et al.; Metab. Clin. Exp. 59, 359 (2010) | (8) V.J. Briscoe, et al.; Expert Opin. Drug Metab. Toxicol. 6, 225 (2010) | (9) F. Liu, et al.; Neurosci. Lett. 557, 90 (2013)





