Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
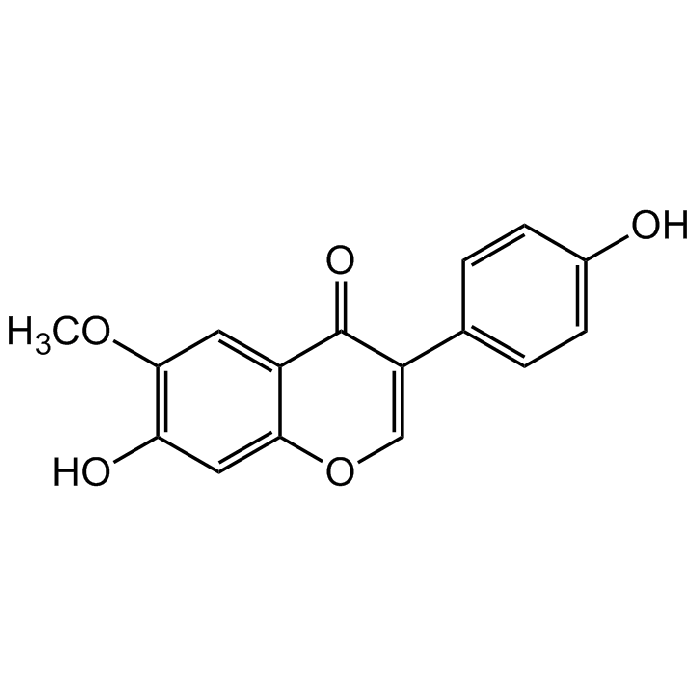
Glycitein
| Product Details | |
|---|---|
| Synonyms | 7-Hydroxy-3-(4-hydroxyphenyl)-6-methoxy-4H-1-benzopyran-4-one; 4',7-Dihydroxy-6-methoxyisoflavone; Glycetein |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H12O5 |
| MW | 284.26 |
| CAS | 40957-83-3 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White powder. |
| Solubility | Sparingly soluble in water, DMSO or DMF (all <1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | DXYUAIFZCFRPTH-UHFFFAOYSA-N |
| Smiles | OC1=C(OC)C=C2C(OC=C(C3=CC=C(O)C=C3)C2=O)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Glycitein is an O-methylated isoflavone. This phytoestrogen is reported to have weak estrogenic activity, displacing estradiol binding at the estrogen receptor in vitro. It suppresses the proliferation of osteoblasts and promotes differentiation from its progenitor. It has also been shown to have antioxidant properties and to inhibit c-Jun N-terminal kinase. It also has been shown to inhibit glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression, or to induce reactive oxygen species-dependent apoptosis and G0/G1 cell cycle arrest through the MAPK/STAT3/NF-κ pathway in human gastric cancer cells.
(1) T.T. Song, et al.; J. Agric. Food Chem. 47, 1607 (1999) | (2) H. Yoshida, et al.; Biosci. Biotechnol. Biochem. 65, 1211 (2001) | (3) A. Gutierrez-Zepeda, et al.; BMC Neurosci. 6, 54 (2005) | (4) K.A. Kang, et al.; Free Radic. Res. 41, 720 (2007) | (5) E.J. Lee, et al.; Chem. Biol. Interact. 185, 18 (2010) | (6) M. Winzer, et al.; Wien. Med. Wochenschr. 160, 446 (2010) | (7) Y.Q. Zang, et al.; Drug Dev. Res. 80, 573 (2019)





