Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
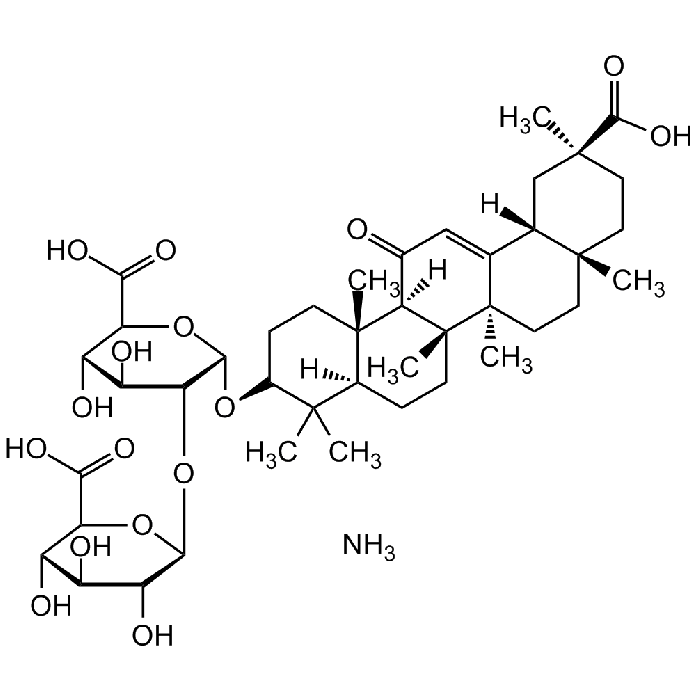
Glycyrrhizic acid ammonium salt
| Product Details | |
|---|---|
| Synonyms | Glycyrrhizin; 3-O-(2-O-β-D-Glucopyranuronosyl-α-D-glucopyranuronosyl)-18β-glycyrrhetinic acid ammonium salt; Monoammonium glycyrrhizinate; NSC 2800; NSC 35348 |
| Product Type | Chemical |
| Properties | |
| Formula |
C42H62O16 . NH3 |
| MW | 839.96 |
| CAS | 53956-04-0 |
| RTECS | LZ6500000 |
| Source/Host Chemicals | Plant |
| Purity Chemicals | ≥98% (Titr.) |
| Appearance | White powder. |
| Solubility | Soluble in DMSO (10mg/ml). Slightly soluble in water (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | ILRKKHJEINIICQ-IERXZFCASA-N |
| Smiles | O=C(O)[C@@H]1[C@@H](O)[C@H](O)[C@@H](O)[C@H](O[C@@H]2[C@@H](O)[C@H](O)[C@@H](C(O)=O)O[C@@H]2O[C@H]3C(C)(C)[C@@](CC[C@]([C@@](CC[C@]4(C)[C@@]5([H])C[C@@](C)(C(O)=O)CC4)(C)C5=C6)(C)[C@]7([H])C6=O)([H])[C@]7(C)CC3)O1.N |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Glycyrrhizic acid is a natural triterpenoid saponin. It is an agonist of the human sweet taste receptor and is used as a flavorant. Glycyrrhizic acid has diverse biological activities, including antioxidant, anti-inflammatory, antidiabetic, anticancer, antimicrobial, hepatoprotective, neuroprotective and antiviral actions. Glycyrrhizic acid has been shown to be a 11-β-hydroxysteroid dehydrogenase inhibitor, a functional inhibitor of HMGB1 and a highly selective and competitive inhibitor of kynurenine aminotransferase 2 (KAT2). It can enhance GLP-1 secretion through TGR5 activation and has an anti-inflammatory effect by attenuating the generation of excessive NO, PGE(2), and ROS and by suppressing the expression of pro-inflammatory genes through the inhibition of NF-κ and PI3K activity. It has anti-angiogenic and apoptosis inducing properties. In addition, due to its amphiphilic nature glycyrrhizic acid is able to form self-associates in aqueous and non-aqueous media, as well as water soluble complexes with a wide range of lipophilic drugs. It has been identified as promising carrier material for anticancer therapy, due to its anti-cancer-related pharmacological activities, such as broad-spectrum anti-cancer ability, resistance to the tissue toxicity caused by chemotherapy and radiation, drug absorption enhancing effects and anti-multidrug resistance (MDR) mechanisms.
(1) R. Pompei, et al.; Experientia 36, 304 (1980) | (2) N.M. Gandhi, et al.; J. Radiat. Res. 45, 461 (2004) | (3) C.Y. Wang, et al.; J. Agric. Food Chem. 59, 7726 (2011) | (4) Y.Y. Chia, et al.; Eur. J. Pharmacol. 677, 197 (2012) | (5) K.J. Kim, et al.; Phytother. Res. 27, 841 (2013) | (6) F.S. Chueh, et al.; Oncol. Rep. 28, 2069 (2012) | (7) E.S. Ramli, et al.; J. Bone Miner. Metab. 31, 262 (2013) | (8) L.J. Ming & A.C. Yin; Nat. Prod. Commun. 8, 415 (2013) (Review) | (9) A. Lau, et al.; Am. J. Nephrol. 40, 84 (2014) | (10) H.S. Cheng, et al.; Nat. Prod. Bioprospect. 4, 325 (2014) | (11) Y. Ohtsu, et al.; Mol. Cell. Endocrinol. 394, 70 (2014) | (12) L. Wang, et al.; Acta Pharmac. Sin. B 5, 310 (2015) | (13) S. Ojha, et al.; Neurotox. Res. 29, 275 (2016) | (14) L.Y. Wang, et al.; Biomed. Pharmacother. 95, 599 (2017) | (15) Z.G. Sun, et al.; Mini Rev. Med. Chem. 19, 826 (2019) (Review) | (16) O.Y. Selyutina & N.E. Polyakov; Int. J. Pharm. 559, 271 (2019) (Review) | (17) Y. Yoshida, et al.; Sci. Rep. 9, 10243 (2019)





